Why use biocides
advertisement

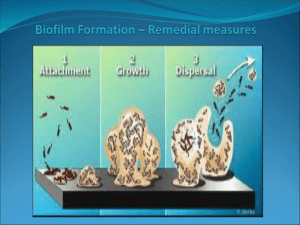
Mirrico Seminar, Kazan, September 2011 Paul Wood and Anna Vatsurina Outline Learnings from an incident Review Risk, Hazard and Exposure Exposure issues Examples of how Dow Microbial Control, controls exposure to biocides Packaging Labelling Safe Removal Transport Lab Safety Storage Dosing Suggestions Conclusions Everyone Learns from an Incident Biocides are not dangerous when handled correctly Chemical Handling: Level of Risk Two factors to determine the risk when handling a chemical • Hazard Type or Consequence of Exposure Corrosive = will cause burns (contact may not be detected immediately) Toxic = a poison Respiratory irritant = like household bleach or ammonia Skin sensitizers = cause “skin allergy” • Exposure Potential or Likelihood of Event How much product ? Probability of an exposure ? Level of Risk Priority in hazard management reduces the exposure potential Hazard x Hazard = HIGH LOW HIGH Risk Safe LOW = x Hazard = HIGH = = x Ethanol Ethanol Exposure Potential Unsafe HIGH Exposure Potential How do we come into contact with a chemical ? There are many routes to exposure Inhalation Skin Ingestion Types of Workplace Exposure Exposure to biocide residues Gloves left lying on the floor Water collecting on top of a drum Contaminated cleaning cloth left in a bin Opening, moving, connecting / disconnecting, emptying apparently “Empty” drums Containers used for hazardous materials without labels Cleaning / repair of contaminated installations Door handles / Doors opened by someone wearing gloves Types of Workplace Exposure Exposure to liquid biocides Spills from damaged packaging, or pipe work Dosing from drums, or pails manually Decanting from drums, or pails into smaller vessels Using samples Collecting samples from the production process Taking samples for Quality Control of raw materials Samples shipped to customers Handling samples in the lab. Exposure Control If there is the potential for exposure Perform a hazard- and safety analysis of the intended task according to your internal rules ( EHS review ) Follow the recommendations on MSDS* and Labels Installations and tools you intend to use for the planned task Industrial Hygiene / Cleanliness and Housekeeping PPE (Personal Protective Equipment): type and control intervals Working Instruction Spill-/Leakage Handling Emergency Procedures Training !!! Appropriate ventilation of the working place / area Safety installations: Safety Showers / Eye rinsing stations Biocide Packaging Packaging has to be designed to be safe Containers Have to be UN approved 3H1/Y1.4/150/** GB/3380 Stringent testing Drop tested Are fitted with tamper evident seals for security reasons Are palletised to prevent damage The number of drums are limited on a pallet to prevent overhang and restrict weight Packaging ergonomics On Site Signs & Labelling Product labelling is one source of information but there are others… Signs placed around the workplace inform operators on which and when personal protective equipment should be worn in specific areas In addition use educational posters as part of an on-going safety education program Personal Protective Equipment ( PPE ) • Helmet • Goggles • Suit • Gloves • Rubber Overshoes Burns caused by not wearing gloves Burns caused by improper removal of gloves Lab Safety To avoid mishandling and accidents Always ensure samples are clearly labelled Warning symbols are visible Store products in clearly labelled cabinets To prevent exposure during use Handle biocides in a fume cupboard Make sure lab technicians wear the correct protective clothing: Gloves Goggles Ensure the area where biocides are handled is kept clean Warehouse Storage Store biocides safely • To maximise the safety of people Training Keep pedestrians separate from • product Safety barriers around offices Special bins for hazardous waste Ventilate warehouse To prevent blind corners, or falling containers Restrict pallet height To avoid mishandling Use painted areas Good lighting Suggestions Be proactive Review the MSDS and label warnings Do a safety review of the job Have a written procedure and follow it Evaluate and inspect the equipment to be used Inspect your Personal Protective Equipment Have the spill deactivation kit equipment and chemicals in place before an incident occurs! Why use biocides ? Essential To: Maintain Efficient Heat Exchange Prevent Corrosion Prevent Plugging of Orifices Prevent Potential Health Concerns Maintain Aesthetic Appearance 18 Why use biocides ? Microbes can be: algae if light reaches the collection basin aerobic bacteria anaerobic bacteria (SRB’s) Moulds and yeast 19 Bio-films Microbes can attach to surfaces and produce slime impairs the heat transfer induces microbial corrosion (MIC) clogs filters, screens, casings and nozzles potentially harbours Legionella pneumophila 20 Biofouling Control – Biofilm Issue A biofilm is a layer of slime that is produced by microbes after they attach to a surface Biofilms serve to protect the microbial community that is underneath it Corrosion and H2S production result from the growth of microbes within a biofilm Removing biofilm is more difficult than preventing the formation of a biofilm Stage 1 Conditioning Layer Stage 2 Bacterial Attachment Stage 3 Slime Formation (EPS Production) Stage 4 Slime Thickening Stage 5 Slime Detachment Examples of “Patchiness” in Bio-films on 316 Stainless Steel Review of the active ingredients used in indistrial water cycles 23 Oxidisers Cl2, HOCl, Br2, HoBr, Chloramaines ; ClO2, H2O2 / Peracetic acid Corrosive Very fast acting Effect is short-lived – no permanance Generate high levels of AoX ( adsorbable organic halides ) 24 Glutaraldehyde 1, 5-pentanedial O O H H Glutaraldehyde Features Quick kill (1-3 hours) under alkaline conditions (pH 7-9) Broad spectrum efficacy Highly effective against SRB, biofilm, and Legionella Readily biodegradable at concentrations < 5-ppm Compatible with dispersants, surfactants and most WT chemicals, including CMIT/MIT Compatible with halogens and other WT additives Does not contain or release formaldehyde Kills via cross-linking proteins in cell wall Glutaraldehyde Limitations Weak efficacy versus fungi and algae Stability with ammonia (NH3) and alkaline pH De-activated by bisulphites Polymerises under alkaline and high temperature conditions (haziness / yellowing) Evaporation (volatilization) potential increases with temperature and /or aeration DBNPA 2,2-dibromo-3-nitrilopropionamide Br H Br C C N N C H O Registered Applications Cooling Water (Re-circulating / Once-through / Open / Closed) Retort Systems, Pasteurizers Reverse Osmosis Membranes Air Scrubbers and Washers Paper Mills Additives/Mineral Slurries Enhanced Oil Recovery Publicly Owned Treatment Works DBNPA Features Extremely fast acting (15 - 60 min) Broad spectrum efficacy Highly effective against biofilm and Legionella Effective at low dose levels Easy to dose liquid Non-corrosive at in-use concentration Low environmental impact Short half-life at more alkaline pH’s Kills via reactions with sulphydryls and disruption of respiration and metabolism DBNPA Limitations Liquid product shelf life is limited (6 months) Weak versus fungi and algae Low solubility in water Incompatible with strong nucleophiles and reducing agents Not UV stable Occasionally referred to as an oxidizer THPS C H 2O H H O H 2C + SO4 2- P C H 2O H H O H 2C 2 tetrakis(hydroxymethyl) phosphonium sulphate THPS Feature Benefit Fast acting, broad spectrum Control of wide range of microorganisms Active against SRB; Algae and Useful for a wide range of industrial Legionella applications Dissolves FeS Reduces FeS related problems such as fouling of equipment Low dosages Cost effective Favourable aquatic toxicity Very low impact on ecology and minimal effect on environment Degradation to inert components Non-foaming Easy to use in high-flow systems No organic solvents Safety in use; completely water miscible THPS Limitations Known to release formaldehyde rapidly (25% of total dose) Cationic properties react with anionic inhibitors Not compatible with oxidizing biocides Unstable at high pH Issues with use of THPS in high calcium waters CMIT / MIT Registered Applications Recirculating Cooling Water (Open and Closed) Air Washers & Industrial Scrubbing Industrial Process Water Brewery Pasteurizers and Can Warmers Industrial Wastewater RO / UF Membranes (non-medical; non-potable) Pulp and Paper Slimicide Additive and Slurry Preservation CMIT / MIT Features Product Feature Broad-spectrum activity Customer Benefit Fast Acting Provides control over bacteria, algae, and fungi with no performance gaps. Effective vs Legionella, SRB, and biofilm Provides immediate control circa 10 minutes Stable over a wide range of pH (<9) and temperature (<40° C) Effective under conditions typically encountered in most processes Clear, water soluble, liquid Fully water soluble at use levels and easy to dose Broad chemical compatibility Compatible with most cooling water and papermill additives and biocides Low use rates Cost effective Biodegradable and does not generate AOX or formaldehyde Environmentally friendly CMIT / MIT Limitations Poor stability above pH 9 and >40º C Poor stability with nucleophiles and reducing agents (sulphides, sulphites, amines,) Perceived weakness versus SRB Slow killing Safe handling concerns / sensitization / burns New solid version will address safety issues Comparison of Biocides for WT Oxidizing Products Cl2/HOCl BCDMH ClO2 DBNPA Glutaraldehyde AM 7287 Sump Buddy UCARCIDE 24, 50 THPS AQUCAR THPS 75 CMIT/MIT KATHON WT Rate of kill Very very fast Very fast Fast Fast Slow Persistence No No Yes Yes Yes Excellent Medium Excellent Good Medium pH range 4 - 7.5 Up to 8.5 Up to 9.5 Up to 9.5 2 to 9 Organisms A,B,F A, B, F biofilm ++ A, B, (F) biofilm +++ A, B B, F, Y, M Biodegradability n.a. Fast (readily biodeg.) Very fast (readily biodeg.) Inherently biodegr. Inherently biodegr. FA release No No No Yes No Thermal resistance Actives Used for Industrial Water Treatment and Paper Biocide Cooling Water Air Wash. RO / UF Paper Slimicide Glutaraldehyde x x x x DBNPA x x x x CTAC x CMIT/MIT x DCOIT x x x x x Spasibo