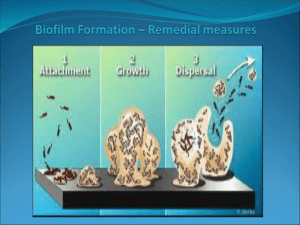
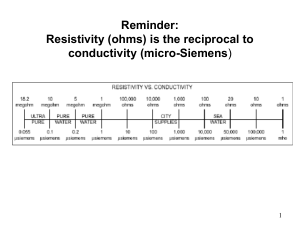
Biofilm in Endoscopy - Surgery Center Coalition
advertisement
BIOFILM AND MEDICAL DEVICES Cheri Ackert-Burr, RN, MSN, CNOR, AGTS Clinical Education Manager Disclosures 1. 2. 3. 4. 5. Successful completion: Participants must complete the entire program and submit the required documentation Conflict of interest: Planners disclose no conflict; the speaker discloses employment with Medivators, thereby declaring a conflict of interest. Commercial company support: Fees are underwritten by education funding provided by Medivators Non-commercial company support: None Alternative Complementary therapy: None Learner Objectives Upon completion of this presentation, participants will be able to: Discuss the concept of biofilm communities in medical devices and in the human body Explain the relationship between biofilm and endoscope/instrument contamination Relate findings from studies and literature to clinical area practice Compare two detergent agents in their effectiveness against biofilm State three best practices of disinfection which are particularly important in biofilm elimination Glossary Exo-polysaccharide (EPS): polymers that are composed of sugar residues and are secreted by a micro-organism into the surrounding environment. Capsular exo-polysaccharides can protect pathogenic bacteria and contribute to their pathogenicity. Glycocalyx: a general term referring to extracellular polymeric material produced by some bacteria composed of carbohydrates, lipids, and proteins. AKA - SLIME Glossary (cont’d) Planktonic: passively floating, drifting, or somewhat motile organisms occurring in a body of water. Sessile: fixed in one place; immobile. Attached by a broad base. Being attached to the substrate or base; not freely moving Vegetative Bacteria: bacteria that are devoid of spores and usually can be readily inactivated by many types of germicides. What is Biofilm? Biofilm: a collection of microorganisms surrounded by the slime they secrete, attached to either an inert or a living surface. Up to 99% of all bacteria live in biofilm communities. Three basic elements to a Biofilm: Microbes Glycocalyx/Exo-Polysaccharide (EPS) Surface (inert or living) Biofilm Lifecycle Biofilm Microbially derived sessile community Irreversible attachment Matrix embedded Altered phenotype Biofilm Formation First observations Communities vs. free-floating Formation in high-shear vs. low-sheer environments Polysaccharides adhere to the surface of medical device and allows other substances to adhere to the surface Where does biofilm live? In industry—biofilms are used to aid in decontaminating water Clogging filters Found in water systems Not just drains, but in the water supply as well—in fact, the higher the water pressure, the more tenaciously the biofilm attaches to the pipe! But where else do they live??? River Rocks On teeth In the Body Colonization Environments Biofilm in the human body > 80% of microbial infections are caused by biofilm – NIH estimate Biofilm in Endoscopes New endoscopes and biofilm Use of endoscopes and biofilm creation Proper processing after each use Studies and Literature Findings Alfa and Howie (2010) Killing bioburden within young vs mature biofilm Glutaraldehyde vs Accelerated Hydrogen Peroxide (AHP) disinfection Studies and Literature Findings Pajkos (2004) Findings of scope contamination studies Evaluation of effectiveness of endoscope cleaning or reprocessing On Medical Devices and Endoscopes Photographic Documentation of Endoscopic Biofilm Scope Tip after Manual Cleaning Distal Tip after Cidex and Alcohol Distal Tip after Peracetic Acid Biofilm Debris in Channel Biofilms Biofilm What has been observed: 99% of bacteria grow as aggregated, sessile communities (biofilm) Biofilm are highly protected and highly resistant to antibacterial treatments (antibiotics and disinfectants) Biofilm are genetically different than bacteria in the planktonic state Biofilm form preferentially in high shear environments Conditions suitable for biofilm development are practically limitless Why we should be afraid, very afraid! Structure and physiology of biofilm confer resistance to antibiotics, disinfectancts, and germicides Experiment: biofilm can adhere to stainless steel, even highly polished SS, within 30 seconds NIH estimates more than 80% of microbial infections in humans caused by biofilm Biofilm in Endoscopes Shortly after its first use, endoscopes develop a conditioning film composed of bodily fluids, proteins, polysaccharides and other components. This alteration of the surface characteristics allows bacteria to commence growth and colonization. Reducing Biofilm Formation Precleaning Cleaning Cleaning Nomenclature Precleaning: Done in the OR, during and immediately after use during a surgical procedure. Should occur at point of use. Can be a wipe down, cleaning spray, or foam to keep instruments moist and to begin the breakdown of bioburden. Manufacturer’s Cleaning Instructions for Use Utilities (eg, type of water, compressed air); Cleaning equipment; Accessories (eg, adaptors) for creating a proper connection between the instruments and equipment, utilities, and cleaning equipment; Accessories for cleaning lumens, ports, and internal parts; Cleaning agents; Lubricants; Processing methods. AORN RP 2014 Cleaning Medical Instruments Manual cleaning Detergents - type Brushes Disassembly Sonic cleaning Detergent Time and temperature Repeat as instructed Washer decontaminator Eliminating Biofilm Destruction of biofilm cell formation Chemical/disinfection reaction with the polysaccharide network Protective shield inhibits cell destruction Hazards of incomplete biofilm removal What are Enzymatic Detergents? Enzymatic Detergents act by reducing the cohesive force within the soil itself Breaks up substances into fine particles which are rinsed away Enzymes act like a pair of scissors “cutting off” soils piece by piece Substrate Specific Different types of Enzymes react to different substances Proteins: #1 concern in a reprocessing room High content of proteins in bodily fluids which need to be removed (blood, tissue, mucous, etc) Protease enzymes break down proteins into their components (amino acids) which are easily removed Substrate Specific Lipids (fats): difficult to penetrate and remove Ex: Olestra is a very large and complex fat molecule Lipase enzymes help break down lipids (hydrolysis). Enzymatic reaction is slow Appropriate surfactants and detergents are generally a bigger player in the effectiveness at removing lipids Substrate Specific • Carbohydrates (starches): very water soluble – a-Amylase enzymes speed up the reaction which breaks starches down into sugars. The sugars are then quickly dissolved into the detergent water and removed Biofilm detaching detergent 6 day growth of a Ps Aeruginosa biofilm Biofilm treated with a bio-detaching detergent Remember the recipes Cleaning Concentration Temperature Time Packaging Handling Storage Each step has specific parameters that must be met Effective Disinfectant Agents Glutaraldehyde Saturated dialdehyde Acetic activated to alkaline to become sporicidal Used in manual or automated processing protocols Peracetic Acid (PAA) Oxidizing agent for disinfection of flexible endoscopes Highly biocidal in the presence of organic soil Improper processing results in regrowth of biofilm Ensuring Patient Safety Shared responsibility Medical instrument care and reprocessing Best practices of cleaning Policies and procedures Training, resources, quality improvement Infection control Orientation and Education Topics Standard Precautions Personal Protection Equipment (PPE) OSHA rules on occupational exposure Reprocessing procedures Mechanisms of disease transmission Maintenance of a safe work environment Safe handling of HLD and sterilants Procedures for waste management Administrative Responsibility Infection Control Guidelines Manufacturer’s Recommendations Identification of problems and lapses in technique Observation of scope storage areas Ensure that reprocessing procedures are followed. Time Out: Test Your Knowledge What are three best practices of medical devices which are particularly important in biofilm elimination? Spray instruments at the point of use before transporting to SPD. Immediately flush the channel with water when contaminated with bioburden. Wipe the external surface of the medical device. When all else fails SGNA AORN IFU Conclusions Every patient deserves a clean endoscope Patient safety is a team responsibility Biofilm elimination is critical Questions Thank You Thank you for your attention. Please be sure you have signed in legibly. Please turn in your evaluations.