Biochemistry 304 2014 Student Edition Membranes
advertisement

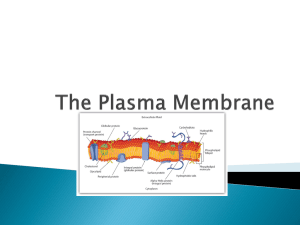
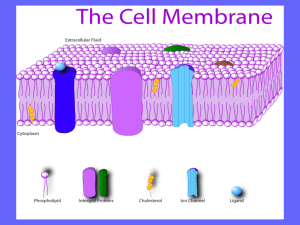
MEMBRANES Student Edition 5/23/13 version Dr. Brad Chazotte 213 Maddox Hall chazotte@campbell.edu Web Site: http://campbell.edu/faculty/chazotte Original material only ©2008-14 B. Chazotte Pharm. 304 Biochemistry Fall 2014 Goals To understand the important roles of biological membrane both within and bounding the cell. To understand the physical forces that give rise to membrane structure and membrane properties. To know the fluid mosaic model of membrane structure and refinements to the original model. To understand motion within membranes and the biological importance of those motions. To be familiar with the types of membrane proteins. To be familiar with membrane fusion, vesicle transport, and related processes such as endocytosis and exocytosis. Significance of Membranes They define the external boundary of cells and regulate the flow of molecules across that boundary. “Compartmentalization” In eukaryotic cells they further define the cell into internal compartments with discrete functions and components. They organize complex reaction sequences and are key to biological energy conservation and communication between cells. The biological properties of membranes derive from their physical properties which in turn are related to their lipid composition. Membrane Properties I Membranes are composed of lipid bilayers that are thermodynamically stabilized in large part by an entropic (hydrophobic) effect. Membranes are: •flexible, self-sealing, and selectively permeable to polar solutes. • not just passive barriers, having an array of specialized proteins for promoting or catalyzing various cellular processes. Membrane Properties II •Impermeable to most polar/charged solutes and permeable to nonpolar compounds •50 to 80 Å thick •Phospholipids form a bilayer w/ nonpolar regions of the lipids facing the inner core and the polar head group face out to the aqueous phase. •Proteins are embedded in the lipids bilayer with their hydrophobic domains interacting with the lipid hydrocarbons. •Protein distribution across the bilayer is asymmetric. Some protein project out of one side, some project through either side of the membrane. “A membrane has sidedness”. •Fluid Mosaic of protein and lipids in the membrane bilayer. Water/Phospholipid Thermodynamics When a nonpolar substance is dissolved in water , it causes and unfavorable organization of water around each molecule. Water molecules orient themselves to maintain intermolecular hydrogen bonds (5-7 kcal/mole each). However, since the water molecules adjacent to the nonpolar molecule have fewer neighboring water molecules there are substantial configurational constraints on the system. Hence there is a decrease in the entropy of the system. In addition, there is no large compensating electrostatic interaction as in the case of ionic or polar molecules. Structures of Amphipathic Lipids in Water ~60Å The shape of the structures is determine thermodynamically from the physical structure (viz. forces & interactions) of the lipid components. Lehninger 2005 Figure 11. 4 Electronmicrograph Voet, Voet & Pratt 2013 Fig 9-15 Lipid Bilayers are in Dynamic Motion: A Computer-Generated Instant Snapshot Water penetration ~15 Å Most “fluid” Least “fluid” •Interior motion with chain C-C bond rotation. •Gives rise to a microviscosity gradient along the chains. •Motion is temperature (energy) dependent Voet, Voet & Pratt 2013 Fig 9-17 Gel & Fluid States of Bilayer Lipids Tm Tm Voet, Voet & Pratt 2013 Fig 9-18 Membrane fluidity is one of the important physiological attributes. Lehninger 2005 Figure 11. 15 The Tm of a bilayer increases with fatty chain length and the degree of saturation of the component fatty acid residues. The rigid planar cholesterol molecule decreases membrane fluidity. Higher amount are found in the plasma membrane Berg, Tymoczko & Stryer 2012 Fig 12. 32 Motions of a Single Phospholipid in a Bilayer •Bilayer is like a 2-D fluid. •In a pure lipid bilayer lateral diffusion is unrestricted •Lateral diffusion is thermodynamically favored whereas transverse diffusion is not. •Barrier to transverse diffusion is reduced via the use of an enzyme; this also allows the process to be controlled. Lehninger 2005 Figure 11. 16 Fluorescence Recovery After Photobleaching Voet, Voet & Pratt 2013 Fig 9-27 FRAP Instrument Lehninger 2005 Figure 11. 17 Phospholipid Translocators & Asymmetric Distribution of Membrane Phospholipids Human Erythrocyte The lipid composition in biological membranes differs in the inner and outer leaflets of the bilayer. There is control over the lipid composition of the bilayer and EACH bilayer leaflet. Phospholipid Translocators: Flippases: translocate primarily aminophospholipids (PE & PS) from outer to inner leaflet & use ATP. (P-Type ATPase). Floppases: move PL from inner to outer leaflet & use ATP (an ABC transporter type). Scramblases: equilibrate PL across both leaflets do not use ATP & are activated by Ca2+. Voet, Voet & Pratt 2013 Fig 9-27 Different composition, then different properties, perhaps different structure, facilitates different FUNCTION. Note: The ER membrane synthesizes nearly all the major classes of lipids required for the production of new cell membranes. Evidence for a Fluid Plasma Membrane: Sendai Virus Fusion Experiment What must happen for the change between initial and final post-fusion states? Set up Initial Final Voet, Voet & Pratt 2013 Fig 9-26 Fluid Mosaic Model of Membrane Structure •Fatty acyl chains form a fluid hydrophobic region. •Integral proteins float in the lipid “sea” held by nonpolar interaction of their amino acid side chains with the lipids. Lehninger 2005 Figure 11. 3 •Proteins and lipids freely diffuse laterally in the bilayer plane but not across the bilayer plane •Lipids and proteins are distributed asymmetrically ACROSS the bilayer •Carbohydrate moieties attached to some proteins and lipids are exposed on the membrane extracellular surface Voet, Voet & Pratt 2013 Fig 9-25 A Cell’s Membranes Have Different Lipid Compositions Composition of Rat Hepatocyte Membranes The compositions of subcellular membranes are different. Different composition→ different structure and/or different function. FYI: PC is a major membrane constituent. Lehninger 2005 Figure 11. 2 Cells control the different compositions of their subcellular membranes. Membrane Proteins Roles: •Catalyze chemical reactions •Mediate nutrient and waste flow across the membrane •Participate in the relay of signal/conditions from the extracellular environment to various internal components. Some Properties: Integral (Intrinsic) proteins are tightly associated with the membrane lipids due to the thermodynamic effect of their hydrophobic interactions. Integral proteins are amphiphiles with the exteriors of the segments in the bilayer having predominately hydrophobic residues, while those segments in the aqueous environment having predominately polar residues on the protein surface . Transmembrane proteins often have α-helical regions that span the bilayer or βsheets. Examples of Membrane Protein Structure •Hydrophobic residues are generally on the surface of a protein in the lipid bilayer and vice-versa outside the bilayer. Aquaporin-O Structure in a Lipid Bilayer Voet, Voet & Pratt 2013 Fig 9.19, 9-20 Human Erythrocyte Glycophorin A Sequence •An α-helix or β-sheet is the protein secondary structure typically used to transverse the membrane bilayer. •Oligosaccharides are typically found on the extracellular side of an integral protein. Bacteriorhodopsin, Hydropathy Plots, and Membrane-spanning Proteins •α-helical membrane spanning segments. •Hydropathy plot can help predict the transmembrane sequences Berg, Tymoczko & Stryer 2012 Table 12.2 Photoreaction Center Lehninger 2005 Figure 11. 9, 11.10 Glycophorin Lehninger 2005 Figure 11. 11 Voet, Voet & Pratt 2013 Fig 9.21 Integral Membrane Protein Types I & II: single transmembrane αhelix III: multiple transmembrane helices in single polypeptide IV: transmembrane domains of several polypeptides form a channel through a membrane. V: protein held to membrane by covalent link to membrane lipid VI: protein has both transmembrane helicies and lipid (GPI) anchor Lehninger 2005 Figure 11. 8 Lipid-Linked Proteins (lipid anchors protein to membrane) Three types of lipid-linked proteins: 1. Prenylated (built from isoprene units) 2. Fatty acylated (myristoylation or palmitoylation) 3. Glycerophosphatidylinositol-linked (phosphotidylinositol glycosidically linked to a linear tetrasaccharide) Lehninger 2005 Figure 11. 14 Voet, Voet & Pratt 2012 Page 263; Figure 9-24 Peripheral (Extrinsic) Proteins Can be dissociated by mild treatment from membrane, e.g. high ionic strength or pH changes. Do not bind lipid hydrophobic regions. Bind at membrane surface to certain lipid head groups or integral proteins. Capable of reversible binding that may vary with conditions. Lehninger 2005 Figure 11. 6 Limitations on Lateral Diffusion in Membranes Original Fluid Mosaic model suggested free lateral diffusion. The reality is more complex due to a number of factors. This is particularly noted in the plasma membrane. •Cytoskeleton •“Fences” and “Corrals” •Lipid Rafts •(Obstructed diffusion) Hop Lateral Diffusion of Lipid Molecules Lehninger 2005 Figure 11. 18 Protein Lateral Diffusion can be Limited in a Plasma Membrane Human Erythrocyte Membrane Cytoskeleton: Illustration Voet, Voet & Pratt 2013 Figure 9-29a,b; 9.31 EM of Human Erythrocyte Protein Mobility Model Membrane Cytoskeleton Microdomains (Rafts) in a Plasma Membrane •Stable associations of sphingolipid & cholesterol •Enriched w/ specific types of membrane proteins. •GPI-linked protein in outer leaflet. •Long chain acyl group on proteins common in the inner leaflet •Can be up to 50% of membrane surface area. Likely role with membrane receptors and signaling proteins! Viral Infection. Structure/function! Lehninger 2005 Figure 11. 20 •Protein in the raft more likely to collide with one another Membrane Fusion Necessary for a variety of cellular processes. Mediated by fusion proteins that are integral membrane proteins responsible for specific recognition and transient local distortion of the bilayer structure. Specific fusion of membranes requires: 1. Recognition of other membrane 2. Close apposition of surfaces 3. Local disruption of bilayer structure w/ fusion of outer leaflets. 4. Bilayers fuse to form single continuous bilayer. 5. Process trigger at appropriate time or via a specific signal. Lehninger 2005 Figure 11. 23 Vesicle Transport & Fusion with Plasma Membrane Eukaryotes: vesicle trafficking – a vesicle buds off one membrane, is transported, and fuses with different membrane. Carries out transfer of lipids and proteins to different membrane. Preserves orientation of integral protein in target membrane as in “parent” membrane Clathrin Coated Vesicles Coated Vesicle Types: Clathrin for transmembrane, GPI & secreted proteins from Golgi to plasma membrane COPI for anterograde & retrograde transport between Golgi compartments Voet , Voet & Pratt 2013 Fig 9-42;9.419a COPII for protein transport ER to Golgi Model for SNARE-Mediated Vesicle Fusion Numerous proteins have been identified that make fusion possible. Proteins must aid in vesicle approach to target membrane. Proteins must aid in the transient bilayer destabilization. Snare Complex Model Voet, Voet & Pratt 2013 Figure 9-46; 9-47 Receptor-Mediated Endocytosis Berg, Tymoczko & Stryer 2002 Fig 12. 40 End of Lecture Text Slide Text Information