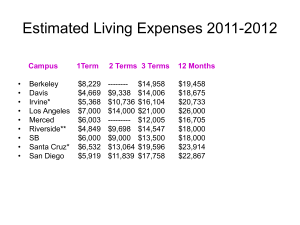
Slides - EveryLife Foundation for Rare Diseases
advertisement

A Third-Party Model for Expanded Access Programs 1. We can deliver large EAPs, far in advance of FDA approval. 2. We can do it in today’s regulatory environment. The ALS Emergency Treatment Fund. Jess Rabourn www.ALSETF.org February 27th, 2014. Washington DC. Hosted by EveryLife Foundation for Rare Diseases Aligning the interests of current patients, industry, and regulators Drug Companies ALS-ETF Regulators Patients Experience, focused in Expanded Access Programs Medical Advisers CRO Network Business Advisory Science Advisory Richard Bedlack, MD, PhD Cato Research Clinical Operations DeNovo Biomarkers Richard Barohn, MD Kaizen Research Manufacturing Control UCSF Terry Heiman-Patterson, MD INC Research Regulatory Affairs Jackson Labs Gerard Kennealy, MD ICON plc Biostatistics Iron Horse Diagnostics Lynn Klein, RN Legal / Liability Decision factors for EAP sponsors Perceived Challenges: Perceived Benefits: •Possibility of adverse events impacting regulatory filing •Fulfillment of unmet patient needs (NEED for OPTIONS) •Regulatory policies that restrict access to these drugs •Collection of additional “real-world” data outside of the clinical trial setting •Risk of impairing enrollment in trials •Development of network of physicians educated on the proper use of the drug •Drain of internal company resources •Different EAP regulations offshore •Generation of data on drug usage patterns to help forecast demand •Possible impact on reimbursement once drug is launched •Establishment of KOL relationships prior to launch Source: 2008-2009 Survey conducted by IDIS via Pharmaceutical Executive Do the factors balance? Commercial Risks Address Patients’ Immediate Treatment Need Additional Regulatory Risks Medical Monitoring Concerns Expense Economic Reward Q: Do the factors balance? A: Depends on the company’s situation For lean companies in poorly understood diseases………….. Big Regulatory Risk Uncontrollable Costs Uncertain Economic Reward 501c3 Public Charity. Intrinsic alignment with patients Allows for: • Regulatory Negotiation, representing patients’ needs first, then company’s – – – – • Charitable Resources – – • Advocate EAP launch early in development cycle Cost recovery Clear agreement on findings of AEs Our own meetings with review division Project financing In-kind, pro-bono, at-cost services Clinic Relationships – – ALSETF providing a service to participating clinics, not the other way around Cost partnership; no profit margin The evolution of expanded access business models? No EAP. Fail Traditional EAP. Not meaningful in size. No NPO engagement. Expensive. Nonprofit 3rd party sponsor. Multi-stakeholder involvement. Little to no cost to drug company How can more EAPs be done at meaningful size? 1. Our target community is dying patients who have no access to clinical trials. 1. Fail enrollment criteria 2. Cannot come frequently to clinic 3. Trial is closed / over-enrolled. Tens of thousands unable to participate in research. Cost Recovery = Scalability These organizations launched scalable humanitarian platforms. Others help in expanding the reach. (individuals, Oxfam, UNICEF) Is this the best we can do? 1. Cost recovery - Expandable Access Program 2. EAP sponsored by 3rd party non-profit partner 3. Managed regulatory risk 4. Minimal cost to drug company LEARNING FROM PATIENTS! *Learning from Patients*: Responder Profiling and Marker Discovery Large EAPs can enable discovery of subgroup markers for more targeted trials, companion diagnostics, swifter marketing approval, and more personalized clinical care. = ALIGNMENT OF INTERESTS Patients / Drug Companies / Regulators (and Researchers & Clinics, too) SUMMARY 1. FDA cannot offer access programs. IND sponsors do. 2. FDA policies already permit what we want to do. 3. A nonprofit 3rd party can make EAP feasible. 4. Early, Expandable EAP for meaningful impact 5. Learning From Patients 6. Not just for ALS! Franchise this to other diseases.