Advanced Lewis Structures
advertisement

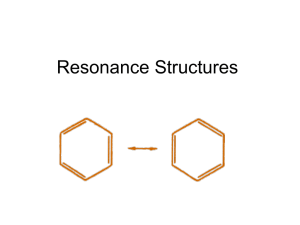
Representing Molecules Resonance Exceptions to the Octet Rule Formal Charge Bond Length •The average distance between two bonded atoms •The distance between nuclei at their minimum potential energy Bond Dissociation Energy • Also called “bond energy” • The energy required to break a bond and form neutral isolated atoms Trends in Bond Energy • C-C vs. C=C vs. C≡C – Higher bond energy for multiple bonds compared to single bonds • Triple bonds are shorter on average than single bonds – There can be a lot of variation in bond length, depending on other bonds for those atoms Bond Energy & Bond Length • Higher bond dissociation energy is linked to lower chemical reactivity • Longer bond lengths lower bond dissociation energies – “Short bonds are strong bonds” Ozone, O3 •Draw a Lewis structure for ozone (the oxygen atoms are in a line) Both structures are equally correct! Resonance Structures • Sometimes, more than one correct Lewis structure can be drawn for a compound – A single Lewis structure may be inadequate for describing the situation – “resonance” structures • Same arrangement of atoms, same numbers of single and multiple bonds • Link the multiple structures with a double headed arrow Resonance • The actual structure is a “blend”, somewhere in between the Lewis structures • Alternating single/double bonds would have different bond lengths – In the real molecule, all the bond lengths are identical! Problem • A problem to try: – Draw resonance structures for the nitrate ion, NO3- Exceptions to the Octet Rule • Elements in row 3 or below on the periodic table can have expanded octets – i.e., they can have more than 8 electrons, because they may use d orbitals in bonding • B, Be may be “electron deficient” – Fewer than 8 electrons around central atom Nonequivalent Lewis Structures • If a molecule or polyatomic ion exceeds the octet rule, or if different skeletal arrangements (i.e. isomers) are possible, non-equivalent Lewis structures may be drawn. • Million dollar question: Which Lewis structure best describes the actual bonding situation? – Use “formal charge” to evaluate Formal charge • FC = # valence electrons – (# of lone pair electrons) – (# bonds) – Calculated for each atom – Sum of formal charges must equal overall charge of species • The most appropriate Lewis structures have: – Lowest formal charges (zero is best) – Negative formal charge on the most electronegative element Calculate formal charges • Which is the most appropriate structure for the molecule CCl2O? Problem • Below are two different Lewis structures for nitrous acid (HNO ). 2 .. .. H-O-N=O: .. .. .. H-N=O .. :O: .. • Which is the better Lewis structure based only on formal charge arguments? ..