atoms = building blocks
advertisement
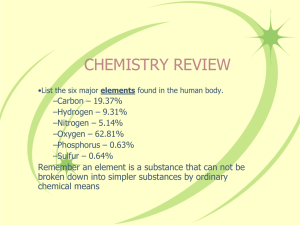
• Matter- the stuff that makes up everything in the universe • Element- A substance that cannot be broken down into other substances by chemical or physical means • Compound- A substance made of two or more elements chemically combined • Mixture- Two or more substances that are mixed together but not chemically combined • Atom- The smallest particle of an element • Molecule- A combination of two or more atoms that are bonded together • ATOMS = BUILDING BLOCKS • Atoms are the basis of chemistry. They are the basis for everything in the Universe. • There are three basic parts of an atom. The parts are the electrons, protons, and neutrons. • The protons and neutrons are always in the center of the atom. • Scientists call the center of the atom the nucleus. • The electrons are always found whizzing around the center in areas called orbitals. Draw in your notebook Draw in your notebook This is a Bohr Model of an atom. • The atom is made up of three parts: • Proton(+)-Small, positively charged particles in the nucleus of an atom • Neutron(0)-Small uncharged particle in the nucleus of an atom • Electron(-)-a tiny, negatively charged, high-energy particle that moves in the space outside the nucleus of an atom Atomic Number • The number of protons in the nucleus of an atom • The identity of an atom is determined by the Atomic # • Helium has 2 Protons, its atomic number is 2 Atomic Number The Periodic Table Atomic weight How to find the # of Protons, Neutrons, & Electron • Protons-Same amount as the atomic # • Electrons- Same as the # of protons • Neutrons- The atomic mass – the atomic # Sodium 11 Protons: 11 Neutrons: 12 Electrons: 11 Na 23 Atomic Mass • The average mass of one atom of an element Valence Electrons • The number of electrons in the outermost orbit of an atom Isotope • An atom with the same number of protons and different number of neutrons (and therefore different atomic masses) • Example: Carbon -12 Carbon -14 Examples of Carbon Isotopes Go to