Orbital Hybridization
advertisement

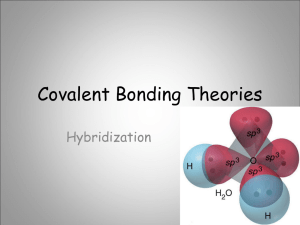
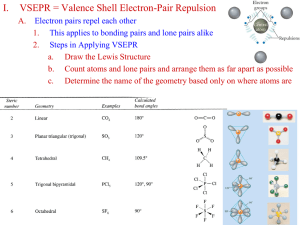
Catalyst Quantum FTW Lecture 2.6 – Orbital Hybridization Today’s Learning Targets • LT 2.7 – I can explain how the overlapping of orbitals with unpaired electrons leads to the creation of covalent bonds and hybrid orbitals. I can explain how sp, sp2, and sp3 orbitals are created and identify a compound as containing one of these types of hybridization. Valence – Bond Theory • While the discussions we have had about bonding has been correct, how can we explain this using quantum mechanics? • Valence Bond Theory – Atoms bond when electron densities are concentrated between the two atoms nuclei. Bonding occurs when valence orbitals of each atom overlap. Quick Write • Draw the Lewis structure for CF4. Predict the electron domain geometry and the molecular geometry of the compound. Consider Carbon… • Carbon’s valence electron configuration looks like: Consider Carbon… • If these form bonds with fluorine as predicted, then 3 of the bonds would have a higher energy than the 4th bond in the s orbital. • The s and p orbitals “mix” in order to form new orbitals that have equal energy • They form sp3 orbitals that allow for bonding to occur Consider Carbon… Orbital Hybridization • Hybrid Orbitals – The orbitals of an atom can “mix” to form new shapes • These orbitals have a new energy level that is a mixture of the two old energy levels. 3 sp Hybrid Orbitals • Occur when 3 p orbitals and 1 s orbital mix • The 4 sp3 orbitals then arrange themselves such that a tetrahedral shape is formed. sp Hybrid Orbitals • Form when the central atom mixes 1 s and 1 p orbital to form 2 sp hybrid orbitals. • Arranged in a linear shape and able to form 2 new bonds • INSERT BeF2 PICTURE 2 sp Hybrid Orbitals • Occur when 2 p and 1 s orbital mix to form new shapes • Hybrid orbitals arrange in a trigonal planar shape and 3 new bonds can form Molecules with Lone Pairs • Central atoms often have lone pairs on them. • These electron pairs are put in their own hybrid orbitals. • Example: H2O Quick Way to Remember Number of Hybrid Orbitals Number of Lone Pairs and/or Bonds for Central Atom Hybridization 2 sp 3 sp2 4 sp3 Class Example • Determine the orbital hybridization of the central atom for NH2 – Table Talk • Determine the orbital hybridization for SO32– 3 dsp and 2 3 d sp Hybridization • For compounds with expanded Octets, d orbitals are hybridized. Number of Lone Pairs and/or Bonds for Central Atom Hybridization 5 dsp3 6 d2sp3 White Board Races Question 1 • What is the hybridization of the carbon atom in CF4? Question 2 • What is the hybridization of the central atom in BI3? Question 3 • What is the hybridization of the central atom in H2O? Question 4 • What is the hybridization of the central atom in NH3? Question 5 • What is the hybridization of the central atom in CO32-? Question 6 • What is the hybridization of the central atom in SF6? Closing Time • Finish reading Chapter 9 and corresponding problems • Pre – Lab for Lab 3 due start of class Thursday/Friday • Lab questions for Labs 2 and 3 due next Wednesday