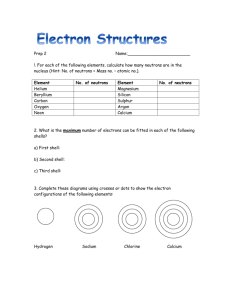
Calculate how many neutrons a phosphorus
advertisement

p. 85, # 1 - 5 p. 85, # 1 - 5 1. Explain how you can determine the number of neutrons an atom has from an atom’s mass number and its atomic number. 1. Atomic number is the number of protons only. Mass number is the total number of protons and neutrons. The number of neutrons is found by subtracting the atomic number from the mass number. 2. 2. Calculate how many neutrons a phosphorus-32 atom has. 32 (mass #) – 15 (atomic #) = 17 neutrons in a phosphorus-32 atom. 3. Name the elements represented by the following symbols. a. Li Lithium d. Br Bromine g. Na Sodium b. Mg Magnesium e. Hg Mercury h. Fe Iron c. Cu Copper f. S Sulfur i. K Potassium p. 85, # 1 - 5 4. Compare the number of valence electrons an oxygen, O, atom has with the number of valence electrons a selenium Se, atom has. Are oxygen and selenium in the same period or group? 4. Both oxygen and selenium have six valence electrons. They are in the same group. 5. Describe how a sodium ion differs from a sodium atom. Which form of sodium is more likely to be found in nature? Explain your reasoning. 5. A sodium atom is uncharged and has one valence electron. A sodium ion is a sodium atom that has lost this valence electron and become Na+. The ion is more often found in nature because the atom easily loses the valence electron to achieve a compete outermost energy level.