Unit 9 (2) - Protons, Neutrons, Electrons Practice Activity
advertisement

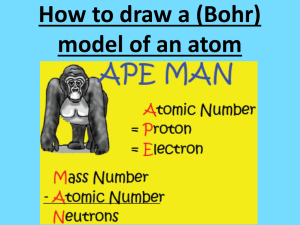
Protons, Neutrons, Electrons How Do We Find All 3? And Where Do We Get These Parts Of The Atoms? So, How Do We Use All These Numbers to Make Sense of What We Have? Protons: can be identified by the ATOMIC NUMBER 6 C 12.011 So, How Do We Use All These Numbers to Make Sense of What We Have? Electrons: can be identified by the ATOMIC NUMBER 6 C 12.011 So, Mr. M…how can we find the Neutrons then? Neutrons: can be found when taking the ATOMIC MASS and subtracting out the ATOMIC NUMBER 6 C 12.011 ATOMIC MASS ATOMIC NUMBER Practice!!!! 12 Mg 24.3 protons neutrons electrons Magnesium 12 Protons 12 Electrons 12 Mg 24.3 Neutrons = Atomic Mass – Atomic Number 24 – 12 = 12 Neutrons Practice 2!!! 17 Cl 35.5 protons electrons neutrons Chlorine 17 Protons 17 Electrons 17 Cl 35.5 Neutrons = Atomic Mass – Atomic Number 36 - 17 = 19 Neutrons Practice 3!!! 107 Bh 262.1 protons electrons neutrons Bohrium 107 Protons 107 Electrons 107 Bh 262.1 Neutrons = Atomic Mass – Atomic Number 262 - 107 = 155 Neutrons Name That Element!!! ?? ?? ?? HELP ME FIND THIS ELEMENT!!! I am a metal with 35 Neutrons...help me find my name!?!?!?! protons Hint: I am missing from either Group 10, 11, or 12. neutrons electrons Dum, Dum-Dum, Dum, Dum... We search the 3 possible groups and we check Atomic Masses – Atomic NUMBERS to see which one gives us 35 Neutrons protons 35 neutrons electrons HAHA!!!! 30 Zn 65.38