File
advertisement

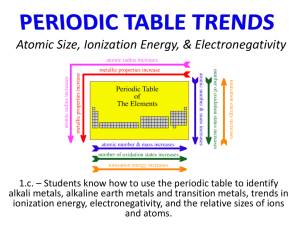
Periodic Law • Do Now: What does an element’s position on the periodic table indicate about its properties? Organizing the Elements • Cu, Ag, Au are three of the oldest known elements. • By the year 1700, only 13 elements had been identified and isolated. • From 1765-1775, five new elements including H, N, and O had been isolated. • As soon as elements were (are) identified, scientists begin to look for similarities and classify them. Classifying Elements • JW Dobereiner published a classification system in 1829. – Elements grouped in triads (3 elements with similar properties) – Couldn’t group all elements using that system • Dmitri Mendeleev published a table of elements in 1869 – Organized in order of increasing atomic mass • Mendeleev When classifying elements, Mendeleev organized according to atomic mass. He left spaces where undiscovered elements would most likely fit. As these elements were discovered, their properties nearly matched those he predicted. His table gained widespread acceptance. Mendeleev’s Table The Periodic Law • In developing the Periodic Table, Mendeleev noticed problems. • Atomic mass order didn’t always match periodic trends. • The table was eventually rearranged according to atomic number. In the modern periodic table, elements are arranged in order of increasing atomic number. • periodic tables Rows or Periods • • • • • • • 7 rows (called periods) on the periodic table Period 1 has 2 elements Period 2 has 8 elements Period 4 has 18 elements Period 6 has 32 elements Each period corresponds to a principle energy level Higher periods have more elements because there are more orbitals in higher energy levels Columns or Groups • Groups have similar properties. • Properties change across a period but those properties repeat as you move from period to period • Periodic Law: when elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. • The properties of an element are similar to those of other elements in the same group. Periodic Law: • When elements are arranged in order of increasing atomic number, there is a of their physical and chemical properties. • • Horizontal rows = are 7 periods • Vertical column = •There (or family) –•Similar physical & chemical prop. –•Identified by number & letter (IA, IIA…) Metals, Nonmetals, Metalloids • 3 broad classes of elements • Across a period, elements become less metallic, and more nonmetallic Metals, Nonmetals, and Metalloids Basic Properties of Elements Metals • 80% of all elements • Conduct heat and electricity • High luster or sheen • Solid at room temp (except Hg) • Ductile • malleable Nonmetals • Most are gases • Some solids • Br is a liquid at room temp. • Properties opposite to those of metals Metalloids • Located on stairstep line • Properties of metals and nonmetals • Very useful because of ability to control properties Metals • 80% of the elements are considered to be metallic • Freshly cut metals have high luster • All metals are solid at room temperatureexcept Hg • Most metals are ductile and malleable (can be drawn into wires and hammered into thin sheets) Nonmetals • MOST nonmetals are gases at room temperature • Some are solids (carbon, sulfur, etc) • One is a liquid- bromine Poor conductors of electricity Exception-carbon Metalloids • “stair step” line separates metals from nonmetals-the elements along this line are known as metalloids • Have properties similar to metals and nonmetals • Chemists do not always agree on which elements to classify as metalloids • http://www.ehow.com/info_8675759_usesmetalloids-industry.html Do Now • Which of the following is least likely to be a metalloid? • A. As • B. Hg • C. Ge • D. Si • E. Sb Do Now • Chemical properties of the elements are defined by the • A. Electrons • B. Ionization energy • C. Protons • D. Neutrons • E. electronegativity Classifying Elements • Squares in the Periodic Table tell us: –Atomic # –Element name-black/solid, gases/red, blue/liquid –Average atomic mass –Vertical column of numbers in the top right corner-number of electrons in each occupied energy level Sort Elements based on Electron Configuration –Noble gases- s and p sublevels full ex: Ar 1s22s22p63s23p6 Representative elements- Group 1A-7A group #=# of electrons in highest occupied energy level Classifying the elements even further…..by group (column) Groups • Every group has the same number of valence electrons • Can be represented by Lewis Dot Diagram • Lewis Dot Diagram – represents the valence electrons by placing dots around the element symbol Lewis Dot Diagrams • Draw the Lewis Diagram for the following: • • • • • • • • Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon • In a chemical reaction, the outermost electrons make first contact – this is why elements with similar electron structures have similar chemical properties Family (Group) Names • Group I - Alkalai metals Alkalai Metals • Highly reactive - found in nature only as compounds • Reactivity increases from the top of the group to the bottom • http://www.periodicvideos.com/ Family Group Names • Group II – Earth metals Group II Earth Metals • Differences in reactivity among the earth metals are shown by the ways they react with water • Increasing reactivity from top to bottom within a group Transition Metals • Transition metals-have electrons in the d orbitals –Inner transition metals- have electrons in the f orbital Family Group Names • Group III – Boron group Group III Boron Group • Aluminum is the most common element in this group and is the most abundant metal in the Earth’s crust Family Group Names • Group IV – Carbon group Group IV Carbon Group • Electrical conductivity increases from top to bottom within a group • Most compounds in your body contain carbon • Compounds of silicon and carbon are extremely hard and used to coat saw blades Family Group Names • Group V – Nitrogen group Group V Nitrogen Group • Nitrogen and Phosphorus are the most common group 5 elements • Nitrogen and phosphorus are used in fertilizers • White phosphorus highly reactive, red phosphorus used to make matches ignite • phosphorus Family Group Names • Group VI – Oxygen group (Chalcogens) Group VI Oxygen Group • Oxygen is the most abundant element in the Earth’s crust • Pure oxygen is flammable • Sulfur was one of the first elements to be discovered – used to produce sulfuric acid • http://www.boston.com/bigpicture/2009/06/ sulfur_mining_in_kawah_ijen.html Family Group Names • Group VII – Halogens Group VII Halogen Family • Highly reactive nonmetals • Chlorine used in pools and bleach • Fluorine used in toothpaste • Thyroid gland needs iodine to work properly , added to table salt Family Group Names • Group VIII – Noble Gases Group VIII Noble Gases • Colorless, odorless gases, extremely Unreactive • Some light bulbs are filled with argon to increase life of the bulb Do Now • A solid element has 2 valence electrons. That element must be • A. A halogen • B. A noble gas • C. A radioactive element • D. an alkalai metal • E. An alkaline earth metal Do Now • Which pair of elements is expected to have the most similar properties? • A. Potassium and lithium • B. Sulfur and phosphorus • C. Silicon and carbon • D. Strontium and barium • E. Fluorine and iodine Periodic Law: • When elements are arranged in order of increasing atomic number, there is a of their physical and chemical properties. • Trends in Atomic Size • The electron “cloud” does not have an edge, it is a probability…so how can we measure how large these atoms are? • Measure more than one at a time • Atomic radius– how we measure the size of an atom–defined as half the distance between 2 nuclei of a diatomic molecule Nucleus Atomic radius Total Distance between 2 nuclei Trends in ELECTRONEGATIVITY • Electronegativity-the tendency for an atom to attract electrons to itself when it is chemically combined to another element • An atom with a large electronegativity means it pulls electrons towards itself Electronegativity, is a measure of the ability of an atom in a molecule to attract electrons to itself. Concept proposed by Linus Pauling 1901-1994 Electronegativity Which is more electronegative? • F or Cl ? • Na or K ? • Sn or I ? • Within a the farther down the group, ` the farther away the electron is from the nucleus, low electronegativity • Within a metals are on the left, they let their electrons go easily- low electronegativity • Nonmetals on the right, they want more electrons – high electronegativity • Summary of Electronegativity trend… • Electronegativity increases from left to right across a period • Electronegativity decreases from top to bottom within a group Trends in DENSITY • increases from top to bottom within a group due to smaller change in atomic size and a large change in atomic mass Trends in Melting points and boiling points of metals • decrease from top to bottom within a group – due to atomic size Melting Points and Boiling points of nonmetals • increase from top to bottom within a group • Due to atomic size/nuclear charge Do Now • The best way to estimate the boiling point of Pd is to • A. Average the bp of Rh and Ag • B. Average the bp of Ni and Pt • C. Average the bp of Ir and Cu • D. Average the bp of Co and Au • E. None of these will work Trends in Metallic character Increases from top to bottom within a group • Decreases from left to right across a period The End !!!!!!!!!!!!!!!!!!!