1_7 - Bohr_ Electronegativity_ and Atomic Radius
advertisement

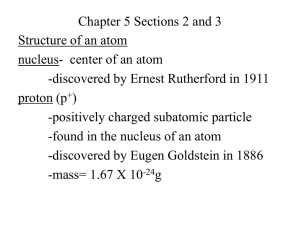
Catalyst 1. Open up the Bohr’s Model educanon video for today’s class. Complete the questions associated with the video. 2. Put your laptop at 45 degrees and place your eyes on me so I know when you’re finished. End Review - Important Point #1 • The atom is mostly empty space. This is why most of the alpha particles went through • The majority of the atom does not have anything in it. Review - Important Point #2 • The atom has very small, but very dense nucleus. • Alpha particles that bounced back were coming into contact with nucleus. • Alpha particles were deflected back because nucleus is positive. Review - Nuclear Model Lecture 1.7 – Bohr, Electronegativity, and Atomic Radius Today’s Learning Targets • 1.6 – I can explain the development of atomic theory incorporating the contributions of Dalton, Thomson, Rutherford, and Bohr. • 1.7 – I can define electronegativity and explain how it relates to the charge of the nucleus and the electron. Furthermore, I can explain how this trend changes as you move throughout the Periodic Table. • 1.8 – I can define atomic/ionic radius and explain how it relates to the charge of the nucleus and the electron. Furthermore, I can explain how this trend changes as you move throughout the Periodic Table. Niels Bohr • Refined Rutherford’s model of the atom with his discovery in 1913. What Bohr Knew • Shooting electricity through the hydrogen excited electrons. • Color produced only for specific values • Problem – If electrons were free to roam, then we should get all sorts of colors Bohr’s Solution • Electrons are not free to roam in the electron cloud, electrons are restricted to orbits or energy levels. The Modern Model Dalton’s atom electron Thompson’s electrons neutron Rutherford’s space and nucleus proton Bohr’s energy levels SUMMARIZE What’s More Attractive? Magnets! How do they work? I. Nuclear Attraction • The negatively charged electrons are attracted towards the positively charged nucleus. • The more the electron “feels” the nucleus. The tighter it is held II. Electronegativity • Electronegativity is the measure of the ability of an atom to attract electrons. III. How does the size of the atom relate to the positive and negative charges in an atom? What do the arrows represent? How does the thickness of the arrows relate to nuclear attraction? IV. Why are the atoms getting bigger? a • As you move from the smallest atom to the largest atom, how does the distance between the valence electrons and the nucleus change? • As you move from the smallest atom to the largest atom, how does the attractive force between the valence electrons and the nucleus change? • What direction does this go on the periodic table? Electronegativity Trends • As you go down a group, the electronegativity of an element decreases. • As you go across a period, the electronegativity of an element increases. Why Electronegativity Decreases Down a Group • As you go down a group more orbits are added. • Electron Shielding – Valence electrons become shielded from the positively charged nucleus as you add more orbits. • Electroneg. decreases because there is a decreased ability of the nucleus to attract electrons because of larger distance. Why Electronegativity Increases Across a Period • As you move across a period you add more protons and electrons within the same orbit. • The larger amount of protons in the nucleus and electrons in orbit show an increased attraction. • This leads to increased electronegativity as you move within a period. Class Example • Order the elements from smallest to largest electronegativity: oxygen, beryllium, lithium, Table Talk • Order the elements from largest to smallest electronegativity: chlorine, bromine, fluorine Stop and Jot • Order the elements from smallest to largest electronegativity: silicon, aluminum, sulfur SUMMARIZE Master Chef - White Board Races White Board Relay Race Problems 1. Which has a larger electronegativity: chlorine or silicon? 2. Which has a smaller electronegativity magnesium or calcium? 3. Put the following elements in order from smallest electronegativity to largest: B, F, N, O. 4. Put the following elements in order from smallest electronegativity to largest: N, As, Bi, P . 5. Why does bromine do a worse job of attracting electrons than fluorine? Atomic Radius • Atomic Radius – The distance from the center of the nucleus to the outermost edge of the electron cloud Outer edge of electron cloud Nucleus Atomic Radius Trends • Atomic radius increases as you go down a group on the Periodic Table. • Atomic radius decreases as you go across a period on the Periodic Table Why Atomic Radius Increases Down a Group • As you add more electron shells to an element, the element becomes “bulkier”. • This means that as you go down a group, more orbits are added, so the radius becomes bigger. Why Atomic Radius Decreases Across a Period • As you move across a period, more protons are added to the nucleus. • Also, more electrons are added within the same orbit. • This means there is a larger positive and negative charge, which results in a higher attraction and a decrease in the radius. SUMMARIZE Master Chef - White Board Races Whiteboard Questions (T/F) 1. Atomic radius is the distance from the center of the nucleus to the first electron orbit. 2. Atomic radius increases across a period and down a group. 3. Atomic radius decreases down a group because you are adding more electron orbits. 4. Atomic radius decreases across a period because there are less electrons in the same orbit. Whiteboard Questions (cont) 5. Order the following from largest to smallest atomic radius: Ca, Se, Ni 6. Order the following from largest to smallest atomic radius: O, Po, S 7. Order the following from smallest to largest atomic radius: Cs, F, Ga Exit Ticket 1. Define atomic radius 2. Define electronegativity. 3. Order from largest to smallest electronegativity: Ca, Se, Ni 4. Order from smallest to largest atomic radius: O, Po, S Rate Yourself • Based on the exit ticket and your current level of understanding, rate yourself 1 – 4 on LT 1.6 - 1.8 Closing Time • Practice 1.7 – Electronegativity and Atomic Radius • UNIT 1 EXAM coming up soon!