Exceptions to the Octet Rule
advertisement
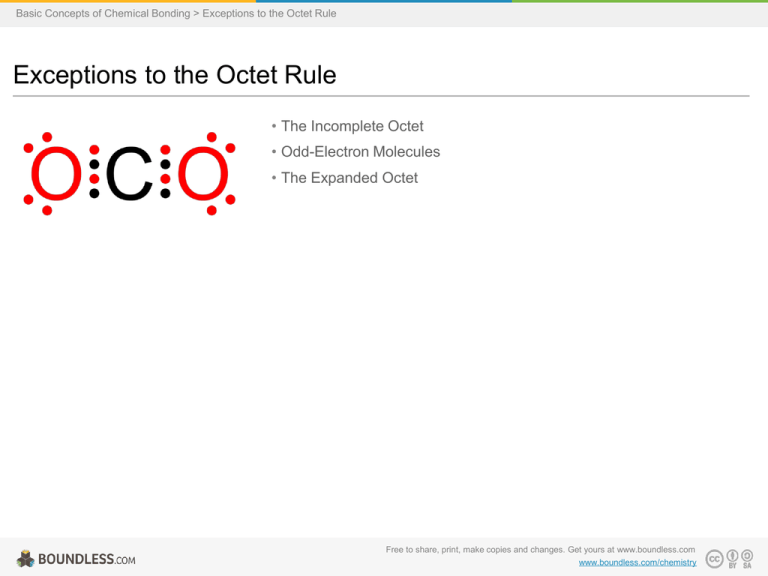
Basic Concepts of Chemical Bonding > Exceptions to the Octet Rule Exceptions to the Octet Rule • The Incomplete Octet • Odd-Electron Molecules • The Expanded Octet Free to share, print, make copies and changes. Get yours at www.boundless.com www.boundless.com/chemistry Basic Concepts of Chemical Bonding > Exceptions to the Octet Rule The Incomplete Octet • The octet rule states that atoms with an atomic number below 20 tend to combine in such a way that they each have eight electrons in their valence shells, giving them the same electronic configuration as a noble gas. • The two elements that most commonly fail to complete an octet are boron and aluminum, both of which readily form compounds in which they have six valence electrons, rather than the usual eight predicted by the octet rule. • While molecules exist that contain atoms with fewer than eight electrons in their valence shell, these compounds are often reactive and can react to form species with eight valence electrons.For example, BF3 will readily bind a fluoride anion to form the BF4- anion, in which boron follows the octet rule. Carbon Dioxide View on Boundless.com Free to share, print, make copies and changes. Get yours at www.boundless.com www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/basic-concepts-of-chemical-bonding-9/exceptions-to-the-octet-rule76/the-incomplete-octet-347-1510 Basic Concepts of Chemical Bonding > Exceptions to the Octet Rule Odd-Electron Molecules • While the majority of compounds formed from atoms below atomic number 20 follow the octet rule, there are many examples of compounds that do not follow the octet rule. • Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule, because the rule requires eight electrons (or two for hydrogen) around each atom. • The most commonly encountered stable species that exist with an odd number of electrons are nitrogen oxides, such as nitric oxide, NO, and nitrogen dioxide, NO2, both of which are free radicals and disobey the octet rule. Nitric Oxide View on Boundless.com Free to share, print, make copies and changes. Get yours at www.boundless.com www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/basic-concepts-of-chemical-bonding-9/exceptions-to-the-octet-rule76/odd-electron-molecules-348-1511 Basic Concepts of Chemical Bonding > Exceptions to the Octet Rule The Expanded Octet • Main group elements that form more bonds than would be predicted by the octet rule are called hypervalent compounds, and have what is known as an expanded octet (more than eight electrons around one atom). • The octet rule can be 'expanded' by some elements by utilizing the d-orbitals found in the third principal energy level and beyond.Sulfur, phosphorus, silicon, and chlorine are common examples of elements forming an expanded octet. • Phosphorus pentachloride, PCl5, and sulfur hexafluoride, SF6, are examples of molecules that deviate from the octet rule by having more than 8 electrons around the central atom (10 for P in PCl5 and 12 for S in SF6). Phosphorus Pentachloride View on Boundless.com Free to share, print, make copies and changes. Get yours at www.boundless.com www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/basic-concepts-of-chemical-bonding-9/exceptions-to-the-octet-rule76/the-expanded-octet-349-1512 Appendix Free to share, print, make copies and changes. Get yours at www.boundless.com Basic Concepts of Chemical Bonding Key terms • atomic number The number, equal to the number of protons in an atom that determines its chemical properties.Symbol: Z • expanded octet A case where an atom shares more than eight electrons with its bonding partners. • free radical Any molecule, ion, or atom that has one or more unpaired electrons.They vary in their reactivity and stability: some are highly reactive and often only occur as transient (i.e., short-lived) species, while others are metastable. • hypervalent molecule A molecule containing an atom from a main group element which deviates from the octet rule by sharing more than eight electrons. • main group element Elements that are not part of the transition metal block in the periodic table. • metastable Of or pertaining to a physical or chemical state that is relatively long-lived, but may decay to a lower energy state when perturbed. • octet rule Atoms lose, gain, or share electrons in order to have a full valence shell of eight electrons.Hydrogen is an exception because it can hold a maximum of two electrons in its valence level. • octet rule Atoms gain, lose, or share electrons with other atoms in order to fill their valence level with eight electrons. • valence electrons The electrons in the outermost (valence) principal energy level of an atom that can participate in the formation of chemical bonds with other atoms. Free to share, print, make copies and changes. Get yours at www.boundless.com Basic Concepts of Chemical Bonding Nitrogen Dioxide Nitrogen dioxide is another stable molecule that disobeys the octet rule.Note the seven electrons around nitrogen.Formal charges and the molecule's resonance structures are indicated. Free to share, print, make copies and changes. Get yours at www.boundless.com Wikibooks. "File:Stickstoffdioxid.svg - Wikibooks, open books for an open world." CC BY-SA 3.0 http://en.wikibooks.org/w/index.php?title=File:Stickstoffdioxid.svg&page=1 View on Boundless.com Basic Concepts of Chemical Bonding Energies of the Highest Occupied Orbitals of the Elements This diagram illustrates the Aufbau rules as they are applied to all the elements.Note especially how the energies of the n th d orbitals fall between the (n+1) s and (n+1) p orbitals.For example, the 3d orbitals begin to fill after the 4s orbital is filled, but before electrons populate the 4p orbitals.A similar relation exists with d- and f-orbitals. Free to share, print, make copies and changes. Get yours at www.boundless.com Steve Lower. "Atomic electron configurations." CC BY-SA http://www.chem1.com/acad/webtext/atoms/atpt-5.html#ORBTAB View on Boundless.com Basic Concepts of Chemical Bonding Boron Trifluoride Lewis Structure Each pair of dots represents a pair of electrons.When placed between two atoms, the electrons are in a bond.A bond can also be drawn as a line between two atoms, also indicating two electrons.Notice that the central boron atom has only 6 electrons in the final Lewis diagram/structure of this molecule. Free to share, print, make copies and changes. Get yours at www.boundless.com Amazon Web Services. "Boundless." CC BY-SA 3.0 http://s3.amazonaws.com/figures.boundless.com/506f0850e4b0dfd51f0b0f57/BF3+and+BF3NH3.png View on Boundless.com Basic Concepts of Chemical Bonding Boron Trifluoride-Ammonia Complex This covalent compound (NH3BF3) shows that boron can have an octet of electrons in its valence level. Free to share, print, make copies and changes. Get yours at www.boundless.com Amazon Web Services. "Boundless." CC BY-SA 3.0 http://s3.amazonaws.com/figures.boundless.com/506f0a32e4b0c20b80855491/BF3NH3.png View on Boundless.com Basic Concepts of Chemical Bonding Sulfur Hexafluoride In the SF6 molecule, the central sulfur atom is bonded to six fluorine atoms, so sulfur has 12 bonding electrons around it.The overall geometry of the molecule is depicted (tetragonal bipyramidal, or octahedral), and bond angles and lengths are highlighted. Free to share, print, make copies and changes. Get yours at www.boundless.com Wikipedia. "Sulfur-hexafluoride-2D-dimensions." CC BY-SA http://en.wikipedia.org/wiki/File:Sulfur-hexafluoride-2D-dimensions.png View on Boundless.com Basic Concepts of Chemical Bonding Carbon Dioxide A Lewis dot diagram for carbon dioxide. Free to share, print, make copies and changes. Get yours at www.boundless.com Wikipedia. "Carbon-dioxide-octet-Lewis-2D." CC BY-SA http://en.wikipedia.org/wiki/File:Carbon-dioxide-octet-Lewis-2D.png View on Boundless.com Basic Concepts of Chemical Bonding Phosphorus Pentachloride In the PCl5 molecule, the central phosphorus atom is bonded to five Cl atoms, thus having 10 bonding electrons and violating the octet rule.The overall geometry of the molecule is depicted (trigonal bipyramidal), and bond angles and lengths are highlighted. Free to share, print, make copies and changes. Get yours at www.boundless.com Wikipedia. "Phosphorus-pentachloride-2D-dimensions." CC BY-SA http://en.wikipedia.org/wiki/File:Phosphorus-pentachloride-2D-dimensions.png View on Boundless.com Basic Concepts of Chemical Bonding Nitric Oxide Nitric oxide, NO, is an example of a stable free radical.It does not obey the octet rule on the nitrogen atom.Each line around the atoms represents a pair of electrons. Free to share, print, make copies and changes. Get yours at www.boundless.com Wikipedia. "File:Nitric oxide.svg - Wikipedia, the free encyclopedia." CC BY-SA http://en.wikipedia.org/w/index.php?title=File:Nitric_oxide.svg&page=1 View on Boundless.com Basic Concepts of Chemical Bonding Which of the following compounds illustrates a deviation from the octet rule? A) BF3 B) H2 C) All of these answers D) AlCl3 Free to share, print, make copies and changes. Get yours at www.boundless.com Basic Concepts of Chemical Bonding Which of the following compounds illustrates a deviation from the octet rule? A) BF3 B) H2 C) All of these answers D) AlCl3 Free to share, print, make copies and changes. Get yours at www.boundless.com Boundless - LO. "Boundless." CC BY-SA 3.0 http://www.boundless.com/ Basic Concepts of Chemical Bonding Nitric oxide violates the octet rule because __________. A) it has more than 8 electrons around the nitrogen atom B) it forms a double bond instead of a single bond C) both its atoms have negative formal charges D) it has an unpaired electron Free to share, print, make copies and changes. Get yours at www.boundless.com Basic Concepts of Chemical Bonding Nitric oxide violates the octet rule because __________. A) it has more than 8 electrons around the nitrogen atom B) it forms a double bond instead of a single bond C) both its atoms have negative formal charges D) it has an unpaired electron Free to share, print, make copies and changes. Get yours at www.boundless.com Boundless - LO. "Boundless." CC BY-SA 3.0 http://www.boundless.com/ Basic Concepts of Chemical Bonding C cannot have an expanded octet because ________. A) it is a second period element B) it has no d orbitals C) Neither of these answers D) Both of these answers Free to share, print, make copies and changes. Get yours at www.boundless.com Basic Concepts of Chemical Bonding C cannot have an expanded octet because ________. A) it is a second period element B) it has no d orbitals C) Neither of these answers D) Both of these answers Free to share, print, make copies and changes. Get yours at www.boundless.com Boundless - LO. "Boundless." CC BY-SA 3.0 http://www.boundless.com/ Basic Concepts of Chemical Bonding Which of the following elements is most likely to form compounds involving an expanded valence shell of electrons? A) P B) O C) Na D) N Free to share, print, make copies and changes. Get yours at www.boundless.com Basic Concepts of Chemical Bonding Which of the following elements is most likely to form compounds involving an expanded valence shell of electrons? A) P B) O C) Na D) N Free to share, print, make copies and changes. Get yours at www.boundless.com Saylor OER. "Chemistry « Saylor.org – Free Online Courses Built by Professors." CC BY 3.0 http://www.saylor.org/majors/Chemistry/ Basic Concepts of Chemical Bonding Attribution • Wikibooks. "General Chemistry/Octet Rule and Exceptions." CC BY-SA 3.0 http://en.wikibooks.org/wiki/General_Chemistry/Octet_Rule_and_Exceptions • Wikipedia. "Octet rule." CC BY-SA 3.0 http://en.wikipedia.org/wiki/Octet_rule • Connexions. "Covalent Bonding and Electron Pair Sharing." CC BY 3.0 http://cnx.org/content/m12584/latest/?collection=col10264/latest • Wikipedia. "valence electrons." CC BY-SA 3.0 http://en.wikipedia.org/wiki/valence+electrons • Wiktionary. "atomic number." CC BY-SA 3.0 http://en.wiktionary.org/wiki/atomic+number • Wikipedia. "Nitrogen dioxide." CC BY-SA 3.0 http://en.wikipedia.org/wiki/Nitrogen_dioxide • Wikipedia. "Nitric oxide." CC BY-SA 3.0 http://en.wikipedia.org/wiki/Nitric_oxide • Wikibooks. "General Chemistry/Octet Rule and Exceptions." CC BY-SA 3.0 http://en.wikibooks.org/wiki/General_Chemistry/Octet_Rule_and_Exceptions • Wiktionary. "free radical." CC BY-SA 3.0 http://en.wiktionary.org/wiki/free+radical • Wiktionary. "octet rule." CC BY-SA 3.0 http://en.wiktionary.org/wiki/octet+rule • Wiktionary. "metastable." CC BY-SA 3.0 http://en.wiktionary.org/wiki/metastable • Wikibooks. "General Chemistry/Octet Rule and Exceptions." CC BY-SA 3.0 http://en.wikibooks.org/wiki/General_Chemistry/Octet_Rule_and_Exceptions • Wikipedia. "Octet rule." CC BY-SA 3.0 http://en.wikipedia.org/wiki/Octet_rule • Wikipedia. "Hypervalent molecule." CC BY-SA 3.0 http://en.wikipedia.org/wiki/Hypervalent_molecule • Steve Lower's Website. "Covalent bonding." CC BY-SA http://www.chem1.com/acad/webtext/chembond/cb03.html#COMP • Wiktionary. "main group element." CC BY-SA 3.0 http://en.wiktionary.org/wiki/main+group+element Free to share, print, make copies and changes. Get yours at www.boundless.com