Half Life - Red Hook Central School District
advertisement
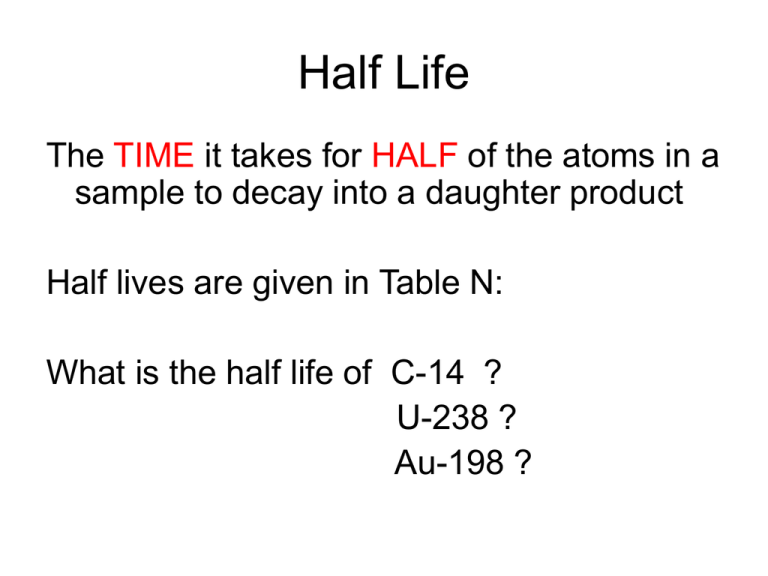
Half Life The TIME it takes for HALF of the atoms in a sample to decay into a daughter product Half lives are given in Table N: What is the half life of C-14 ? U-238 ? Au-198 ? Half Life The shorter the half life, the more unstable the element is, and the faster it decays The longer the half life, the more stable an element is, and the slower it decays Half Life = 8.07 days Half life problems Cr-51 is unstable. If you have 52 grams of Cr-51 in July 1, how many grams will you have on July 28? Half life problems Answer: First look up the half life in Table N = 28 days Since the time it takes for HALF of the atoms to decay is 28 days, on July 28 only HALF of the atoms will be left. Half of 52 grams is 26 grams Cr-51 half life = 28 days How much of the 52 grams will be remaining after 168 days? Day: Half lives: 0 28 56 74 112 140 168 0 1 2 3 4 5 6 grams: 52 26 13 6.5 3.25 1.63 .815 If you had 52g to start, and ended up with .813g after 168 days, what fraction of the original amount remains? 168 days = 6 half lives ½ remains after 1 half life ¼ after 2 half lives 1/8 after 3 half lives 1/16 after 4 half lives 1/32 after 5 half lives 1/64 after 6 half lives If you have .75 g of Cr-51 remaining after 168 days, how much was in the original sample? Days 168 140 112 74 Grams .75 1.5 3 6 56 12 28 0 24 48











