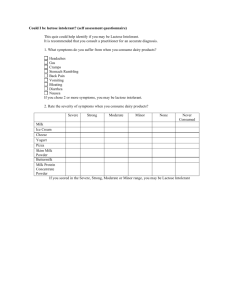
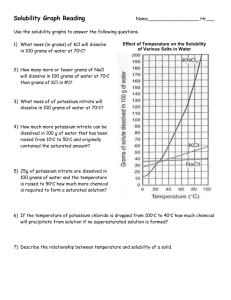
Solutions WSv1
advertisement

Chemistry 20 Calculating Concentrations of Solutions Name:_________________ Due: Wednesday, October 30 Use the formulas from your notes, show ALL your work and make sure your answer is in correct significant digits for full marks. 1) A delicious cake weighs 2.35 kg and contains 200 grams of sugar. What is the percent weight by weight concentration of sugar in this cake? 2) For a lab, you are instructed to add 1.70 grams of zinc nitrate to 300 mL of water. Using your solubility table, determine if this compound is soluble, and if it is, calculate the amount concentration (in mol/L) of the resulting solution. 3) Later in the lab, your procedure tells you to add 0.66 grams of lead (II) sulfate to 250 mL of water. Using your solubility table, determine if this compound is soluble, and if it is, calculate the amount concentration (in mol/L) of the resulting solution. 4) A 4.00 Litre jug of skim milk contains 195.0 grams of lactose. a) What is the percent weight by volume (g/mL) concentration of lactose in this jug of milk? b) If a lactose intolerant person can only handle 7.50 grams of lactose in one day, what is the maximum volume of skim milk they could drink before becoming sick? Express your answer in mL. 5) Fish require a minimum oxygen concentration of 4.60 ppm in their water to survive. What is the minimum mass of oxygen that must be dissolved in a 55.0 L fish tank to ensure the fish survive? Express your answer in mg. 6) One of the tests police use to determine if someone is intoxicated is to measure their Blood Alcohol Concentration (BAC). This is the same as the percent volume by volume (% V/V) concentration of ethanol in a person’s blood stream. If the average human body contains 5.60 L of blood, and a person consumed enough alcohol to have 0.950 mL of ethanol in their bloodstream, what would be their BAC?