Chapter 14 Kinetics
advertisement

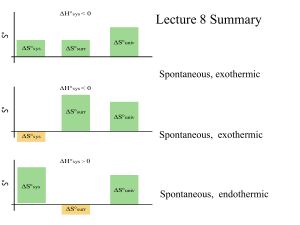
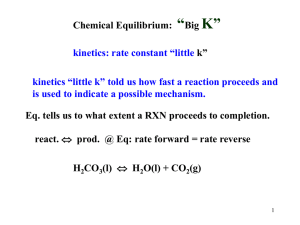
Chapter 14 Chemical Kinetics Chemical Kinetics • Study of rxn rates (how fast rxn progresses) – Measured in [X]/t • Also deals with reaction mechanisms – Steps rxn goes through to move from reactants to products Factors That Influence Reaction Rate Under a specific set of conditions, every reaction has its own characteristic rate, which depends upon the chemical nature of the reactants. Four factors can be controlled during the reaction: 1. 2. 3. 4. Concentration - molecules must collide to react; Physical state - molecules must mix to collide; Temperature - molecules must collide with enough energy to react; The use of a catalyst. Expressing Rxn Rates • Rates are expressed in some unit quantity per time – SI units for rates of distance (speed) are in m/s – Chemical rxn rate units are M/s • Consider the rxn A→B – • Rxn rates given can be initial, instantaneous, or average Expressing Rate aA 1 rate = a + [A] t bB cC 1 = - b + [B] t dD 1 = + c [C] t 1 [D] d t = + The numerical value of the rate depends upon the substance that serves as the reference. The rest is relative to the balanced chemical equation. Practice PROBLEM: Determine the average rxn rate over the course of the entire experiment for the data listed in Table 16.1 PROBLEM: (a) Balance the following equation and express the reaction rate in terms of the change in concentration with time for each substance: NO(g) + O2(g) → N2O3(g). (b) How fast is [O2] decreasing when [NO] is decreasing at a rate of 1.60x10-4 M/s? The Rate Law • The rate law governs the progress of a given rxn. • For a general rxn: aA + bB → cC + dD – The rate law is given by the equation below: – Rate = k[A]x[B]y, • k – rate constant; x & y are rxn orders wrt A & B • All components of a rxn’s rate law must be determined experimentally – Measure physical quantity that you can relate to the concentration of a reactant either @ a specific instant (initial rate method) or over time (integrated rate law) Determining Rate Laws • The Initial rate method – Used to determine rxn orders experimentally – Measure initial rate of rxn @ different reactant concentrations – Data is listed in a table – Ratio data, in general rate law form, from 2 lines in the table to determine order of each reactant in rxn • Choose lines where conc. reactant in question changes and conc. of all other reactants stays the same Practice 2NO(g) + O2(g) Experiment 2NO2(g) Initial Reactant Concentrations (mol/L) O2 NO Initial Rate (mol/L*s) 1 1.10x10-2 1.30x10-2 3.21x10-3 2 2.20x10-2 1.30x10-2 6.40x10-3 3 1.10x10-2 2.60x10-2 12.8x10-3 4 3.30x10-2 1.30x10-2 9.60x10-3 5 1.10x10-2 3.90x10-2 28.8x10-3 1. Determine the general rate law for the rxn. 2. Calculate the rate constant for experiment 2. The Rate Constant • Specific for a particular rxn at a particular temperature, within experimental error • Units for k tell you the overall rxn order – Remember units for rate are M/time – Units for [A]x are Mx – For a rxn with an overall order R, the unit for k can be found by The Integrated Rate Law • Can be used for 2 reasons 1. Determine reactant concentration after an elapsed time--- must know order of reactant, rate constant, correct formula 2. Determine rxn order for a specific reactant--must graph different quantities vs. time and see which gives most linear plot • Can only be used for 0, 1st, and 2nd order rates Integrated Rate Law Formulas & Plots Practice PROBLEM: At 250C, hydrogen iodide (HI) breaks down ver slowly to hydrogen and iodine: rate = k[HI]2.The rate constant @ 250C is 2.4x10-21 L/mols. It 0.0100 mol of HI is place in a 1.00L container, how long will it take for the concentration of HI to reach 0.00900M (10% reacted)? PROBLEM: Determine the rxn order for N2O5 using the graphical data given Reaction Half-Life • Time required for reactant concentration to reach ½ its initial value The Arrhenius Equation • Describes the relationship between temperature and rxn rate where k is the kinetic rate constant at T Ea is the activation energy R is the energy gas constant T is the Kelvin temperature A is the collision frequency factor • • • • Higher T Larger k Increased/faster rate Smaller Ea Larger k Increased/faster rate Lower Ea (or T) Smaller k Decreased/slower rate A is related to both the collision frequency an orientation probability factor (dependent on structural complexity) Activation Energy • Energy that must be overcome for reactants to form products – All rxns regardless of initial and final energies have Ea > 0 – Some bonds must break and new bonds must form – Reactant molecules gain this energy through collisions with one another – Increasing temperature increases rate as # collisions and energy of collisions increase Practice Reaction Energy Diagrams • Used to depict changes reactant molecules undergo to form products Reaction Mechanism • Sequence of single rxn steps that sum to the overall rxn • It is impossible to prove rxn mechanism experimentally • Rxn energy diagrams can elucidate # steps in a mechanism • Steps in a mechanism for an overall rxn are elementary steps in which the coefficients of each reactant denote the reaction rate order wrt the reactant • The sum of all reactant coefficients in an elementary step denote the molecularity of the step • The higher the molecularity of an elementary step, the slower its rate 2NO2(g) + F2(g) → 2NO2F(g) • Experimental rate law determined: – rate = k[NO2][F2] • Accepted mechanism: – NO2(g)+ F2(g) → NO2F(g)+ F(g) – NO2(g) + F(g) → NO2F(g) Correlating Mechanism w/ Rate Law • For a mechanism to be reasonable, its elementary steps must meet 3 criteria: 1. Elementary steps must add up to overall balanced eqtn 2. Elementary steps must be physically reasonable (usu. bi- or lower molecularity) 3. Mechanism must correlate with the rate law • • Overall rate law is usually equivalent to the slowest step’s (the rate limiting step, RLS) rate law RLS can be picked out in a rxn energy diagram and predicted in a mechanism Practice • The rxn and rate law for the decomposition of dinitrogen pentoxide are 2N2O5(g)→ 4NO2(g) + O2(g) rate = k[N2O5]2 and the rxn energy diagram is given above. Which of the following mechanisms is most likely? A. One-step collision C. 2N2O5(g) → N4O10(g) [fast] N4O10(g) → 4NO2(g) + O2(g) [slow] B. 2N2O5(g) → N4O10(g) [slow] N4O10(g) → 4NO2(g) + O2(g) [fast] Catalysis • Increasing rxn rate by adding a catalyst • Catalyst function: – Lowers Ea increases k without being consumed or changing product amount • Usually lowers Ea by providing a different mechanism