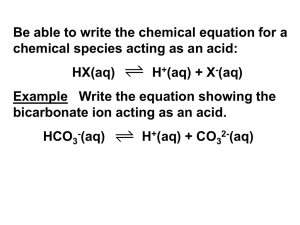Document
advertisement

Chapter 19 Acids, Bases, and Salts Anything in black letters = write it in your notes (‘knowts’) 19.1 – Acid-Base Theories Acids Taste sour Dissolve active metals to produce hydrogen gas Turns litmus paper RED Bases Taste bitter Feels slippery on skin (dissolves oils on skin) Turns litmus paper BLUE Have you seen the litmus paper yet?? These are experimental definitions, they do not explain (theory) how an acid is different from a base. Arrhenius defined an acid and base theoretically. Svante Arrhenius (1857 – 1927) First, a vocab word… Dissociate to split or separate from another Arrhenius Definition (~1887) ACID – substance that dissociates in water to form hydrogen ions (H+). HCl (aq) H+ (aq) + Cl- (aq) BASE – substance that dissociates in water to form hydroxide ions (OH-). NaOH (aq) Na+ (aq) + OH- (aq) When an acid is placed in water, H+ ions are produced. Hydrogen ions can also be thought of as H3O+ ions. H3O+ = hydronium ion HCl (aq) H+ (aq) + Cl- (aq) or equivalently, HCl + H 2O H 3O + + Cl- Brønsted-Lowry Definition (~1923) ACID – donates H+ BASE – accepts H+ Johannes Bronstad (1879 – 1947) Thomas Lowry (1874 – 1936) B-L definition covers more examples than the Arrhenius definition. ammonia ammonium ion water donates a H+ and so is a B-L acid ammonia accepts a H+ and so is a B-L base Conjugate Acid – formed when a base accepts a H+ Conjugate Base – formed when an acid donates a H+ EXAMPLES… ACIDS donate H+, BASES accept H+ label each as acid, base, c. acid, c. base HCO3- + acid HF + + acid + OH- + base + H 2O base HCO3- base + c. base F- H 3O + + c. acid + H2CO3 c. base + c. acid HCO3- acid CO3-2 H2O + CO3-2 c. acid + c. base Amphoteric – substance that can be an acid or a base – depending on what it reacts with. Water is amphoteric ACIDS donate H+, BASES accept H+ label each reactant as an acid and base, label the products as conjugate acids or conjugate bases. HNO3 + H2O H3O+ + NO3- CH3COOH + H2O H3O+ + CH3COONH3 + H2O NH4+ + OHH2O + CH3COO- CH3COOH + OH- Lewis Acids and Bases (not covered) Acid/Base Indicators Litmus Acid – red, Base – blue, Neutral - colorless Phenolphthalein Acid – colorless, Base – pink, Neutral - colorless Cabbage Acid – red/pink, Base – yellow/green, Neutral – blue/purple Assignment: Chapter 19 Worksheet #1 (FRONT) 19.2 – Hydrogen Ions and Acidity Molarity (M) – unit used to express the concentration of a solution mol solute (mol) Molarity = liters of soln (L) anything in [brackets] means the concentration in molarity [H+] = ‘the hydrogen ion concentration’ [OH-] = ‘the hydroxide ion concentration’ Self-Ionization of Water Water ionizes to produce a small amount of H+ and OH- ions. H2O H+ + OH- In pure water at 25̊C [H+] = [OH-] = 1 x 10-7 M Ion-product constant for water (Kw) Kw = [H+][OH-] = 1.0 x 10-14 remember…anything in [brackets] represents the concentration in molarity A solution is acidic if [H+] > 1.0 x 10-7 M …or if the pH of the solution is below 7 Just as the mole was used to simplify large numbers of atoms, pH is used to simplify small concentration numbers pH = ‘power of the hydrogen ion’ pH = -log[H+] Instead of writing out numbers like these… [H+] = 1 x 10-7 M [H+] = 2.4 x 10-4 M pH = 7.00 [H+] = 7.3 x 10-10 M pH = 9.14 pH = 3.62 we can write number like these Instead of saying “This solution has a hydronium ion concentration of 2.4 x 10-4 M”. We can just say “This solution has a pH of 3.62”. Not only is pH an easier number to talk about, pH is understood by most people, whereas molarity is not. The pH scale is used to describe how acidic or basic (alkaline) a substance is. Examples Pure water has [H+] = 1.00 x 10-7 M The pH of water would be pH = -log[H+] pH = - log [1.00 x 10-7] pH = 7 Examples [H+] = 2.3 x 10-5 M. Calculate the pH. pH = - log [H+] pH = - log [2.3 x 10-5] pH = 4.64 Examples [H+] = 1.0 x 10-5 M. Calculate the pH. pH = - log [H+] pH = - log [1.0 x 10-5] pH = 5.0 Examples pH = 4.2. Calculate [H+] pH = - log [H+] -4.2 = log [H+] 10-4.2 = 10log [H+] 10-4.2 = 6.31 x 10-5 M = [H+] Summary of pH Acidic Neutral [H+] 100 M 10-7 M pH 0 7 14 10-7 M 100 M [OH-] 10-14 M pH = - log [H+] Basic 10-14 M [H+] = 10-pH Kw = [H+][OH-] = 1.0 x 10-14 19.3 – Strengths of Acids and Bases Strong acids & bases completely dissociate (split into ions) in water; Weak acids & bases partly dissociate and only form a small amount of ions. HCl is a strong acid, HCl (aq) H+ (aq) + Cl- (aq) it completely ionizes Concentration Initial at Equilibrium [HCl] [H3O+] [Cl-] 0.10 M 0 0 0 0.10 M 0.10 M CH3COOH is a weak acid, CH3COOH (aq) CH3COO- (aq) + H+ (aq) it forms a small amount of ions Concentration Initial at Equilibrium [CH3COOH] 0.10 M 0.0987 M [CH3COO-] [H3O+] 0 0 1.34 x 10-3 M 1.34 x 10-3 M 0.00134 M 0.00134 M Chemical Equilibrium – occurs when forward rxn rate equals reverse rxn rate; dynamic CH3COOH + H2O CH3COO- + H3O+ Le Chatelier’s Principle – at equilibrium, a rxn will shift forward or backward in response to any change in conditions (temp, pressure, concentration) Increase [CH3COOH], rxn shifts the rxn to the right. Increase [CH3COO-], rxn hifts rxn to the left. Le Chatelier’s Principle Example 1 CH3COOH + H2O CH3COO- + H3O+ 1. Add methyl orange to acetic acid; divide into 3’s red pH 4.0, orange pH 5.0, yellow pH 6.0 2. Add Na+CH3COO- to acetic acid Doing this, increases [CH3COO-] and causes a shift in the rxn to the left; increasing the pH. 3. Add NaOH to the acetic acid Doing this, decreases [H3O+] causing a shift in the rxn to the left; increasing the pH. Le Chatelier’s Principle Example 2 NaCl (aq) Na+ + Cl- What will happen when drops of HCl (aq) are added to a saturated solution of NaCl? HCl (aq) H+ + Cl- the rxn will shift to the left because the [Cl-] concentration increased. Le Chatelier’s Principle Example 3 Co(H2O)6+2 (aq) + 4Cl- (aq) + heat CoCl4-2 (aq) + 6H2O 1. Add ~ 3 mL of conc. HCl to about 2 mL of 0.1 M CoCl2 2. Add water to reverse the rxn 3. Add ~ 2 mL of 0.1M AgNO3(aq). Ag+ (aq) + Cl- (aq) AgCl (s) Buffer – solution that maintains a fairly stable pH when small amounts of acid or base are added. Buffer solutions consist of a weak acid and its conjugate base or a weak base and its conjugate acid. Acetic Acid/Acetate Ion Buffer CH3COO- CH3COOH The acetate ion reacts with any added acid. CH3COO- + H+ CH3COOH The acetic acid reacts with any added base. CH3COOH + OH- CH3COO- + H2O Universal pH Indicator Color Chart Blood is buffered at pH b/w 7.35 - 7.45. This is done mainly by the carbonic acid/bicarbonate buffer. Carbonic acid (H2CO3) neutralizes added bases. Bicarbonate ion (HCO3-) neutralizes added acids. H2CO3 (aq) H2O + CO2 Hold your breath, CO2 level builds up in blood, causing [H2CO3] ↑, pH ↓ Hyperventilate, CO2 level drops in blood, causing [H2CO3] ↓, pH ↑ Questions What determines if an acid or base is strong or weak? What occurs during chemical equilibrium? Describe Le Chateliers Principle. CH3COO- + H+ CH3COOH Which direction will the rxn shift if [CH3COO-] increases? What would happen to the pH of the solution? What is a buffer? What two things must a buffer contain? How does a buffer work? Le Chatelier’s Principle and Reversible Reactions ANALYSIS Part 1 1. (a) H2SO4 caused the solution to turn from yellow to orange. (b) Adding H2SO4 caused the reaction to shift to the right. (c) Adding NaOH caused the reaction to shift to the left. Part 2 1. (a) Fe+3 (ferric or iron(III)) & NO3-1 (nitrate) (b) K+1 (potassium) & SCN-1 (thiocyanate) (c) The Fe(SCN)+2 caused the solution to turn dark red. (d) Adding Fe3(SO4)2 increased the concentration of Fe(SCN)+2. (e) Adding Fe(NO3)3 increased the concentration of Fe(SCN)+2. (f) Adding NaOH decreased [Fe(SCN)+2] (g) Reducing [SCN-] caused the rxn to shift to the left (yellow) 19.4 – Neutralization Reactions Neutralization Rxn – complete rxn of a strong base with a strong acid A neutralization rxn will produce a salt and water. Acid HCl + Base + NaOH Salt + H2O NaCl + H2O Titration – determining the concentration of an unknown solution using a solution whose concentration is known. Standard – solution that has a known concentration. Equivalence Point – point where the amount of acid equals the amount of base End Point – point where the indicator changes color ASSIGNMENT: Read Section 19.4 (p. 672 – 675) Answer Questions #35-43 EXAMPLE 10.0 mL of 0.5 M HCl solution is added to 20.0 mL of NaOH of unknown concentration. What is the concentration of the NaOH? HCl + NaOH 0.5 M 10.0 mL xM 20.0 mL NaCl + H2O Since the reaction of HCl and NaOH is 1:1 and twice the volume of NaOH was used, the NaOH must half as strong as HCl; [0.25 M]. EXAMPLE What volume of 0.10 M KOH is required to neutralize 20.0 mL of 0.20 M H2SO4 solution? H2SO4 + 2KOH 0.20 M 0.10 M 20.0 mL x mL K2SO4 + 2H2O Since KOH requires twice as many moles as H2SO4, you should double your answer. 19.5 – Salts in Solution not covered… Write the balanced chemical equation for each neutralization reaction Sulfuric acid + magnesium hydroxide Phosphoric acid + calcium hydroxide Nitric acid + ammonium hydroxide Chapter 19 Quiz #2 1. What color will litmus paper be in an acidic solution? 2. What color will phenolphthalein indicator be in an basic solution? 3. What does [H+] mean? 4. What two products are always formed in an acid-base neutralization reaction? 5. Explain the difference between a strong acid and a weak acid. 6. Explain why the pH of pure water is 7.00 7. What is a buffer? 8. How is the molarity of a solution calculated? 9. A student titrated 10.0 mL of an HCl solution. The titration required 23.3 mL of 0.24M NaOH solution. a. Which solution was the standard? b. Which solution was more concentrated? c. Convert both volumes to liters d. Calculate the number of moles of NaOH that reacted. e. Calculate the number of moles of HCl that reacted. f. Calculate the molarity of the HCl solution. 10. Calculate the pH of solutions with the following hydrogen ion concentrations. a. [H+] = 1.23 x 10-4M b. [H+] = 3.42 x 10-7M 11. Calculate the hydrogen ion concentrations of solutions with the given pH. a. pH = 3.14 b. pH = 9.2
![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)