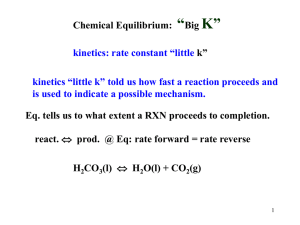
Lecture 9: Gibbs Free Energy
advertisement

°sys < 0 S°sys S°surr Lecture 8 Summary S°univ Spontaneous, exothermic °sys < 0 S°surr S°univ Spontaneous, exothermic S°sys °sys > 0 S°sys S°univ S°surr Spontaneous, endothermic Lecture 9: Gibbs Free Energy G Reading: Zumdahl 10.7, 10.9 Outline Defining the Gibbs Free Energy (G) Calculating G (several ways to) Pictorial Representation of G Defining G Recall, the second law of thermodynamics: Suniv = Stotal = Ssys + Ssurr Also recall: Ssurr = -Hsys/T Substituting, Stotal = Ssys +-H S /T syssurr Multipling all by (-T) gives -TStotal = -TSsys+ Hsys We then define: G = -TStotal Substituting G = -TSsys+ Hsys Giving finally G = H - TS G = The Gibbs Free Energy @const P G and Spontaneous Processes Recall from the second law the conditions of spontaneity: Three possibilities: If Suniv > 0…..process is spontaneous If Suniv < 0…..process is spontaneous in opposite direction. If Suniv = 0….equilibrium In our derivation of G, we divided by -T; therefore, the direction of the inequality relative to entropy is now reversed. Three possibilities in terms of S: If Suniv > 0…..process is spontaneous. If Suniv < 0…..process is spontaneous in opposite direction. If Suniv = 0…. system is in equilibrium. Three possibilities in terms of G: If G < 0…..process is spontaneous. If G > 0…..process is spontaneous in opposite direction. If G = 0….system is in equilibrium Spontaneous Processes: temperature dependence Note that G is composed of both H and S terms G = H - TS A reaction is spontaneous if G < 0. So, If H < 0 and S > 0….spontaneous at all T If H > 0 and S < 0….not spontaneous at any T If H < 0 and S < 0…. becomes spontaneous at low T If H > 0 and S > 0….becomes spontaneous at high T Example: At what T is the following reaction spontaneous? Br2(l) Br2(g) where H° = 30.91 kJ/mol, S° = 93.2 J/mol.K G° = H° - TS° Try 298 K just to see result at standard conditions G° = H° - TS° G° = 30.91 kJ/mol - (298K)(93.2 J/mol.K) G° = (30.91 - 27.78) kJ/mol = 3.13 kJ/mol > 0 Not spontaneous at 298 K At what T then does the process become spontaneous? G° = H° - TS° = 0 T = /S T = (30.91 kJ/mol) /(93.2 J/mol.K) T = 331.65 K Just like our previous calculation Calculating G° In our previous example, we needed to determine H°rxn and S°rxn separately to determine G°rxn But ∆ G is a state function; therefore, we can use known G° to determine G°rxn using: Grxn = Gprod. - Greact. Standard G of Formation: Gf° Like Hf° and S°, the standard Gibbs free energy of formation Gf° is defined as the “change in free energy that accompanies the formation of 1 mole of that substance for its constituent elements with all reactants and products in their standard state.” As for Hf° , Gf° = 0 for an element in its standard state: Example: Gf° (O2(g)) = 0 Example • Determine the G°rxn for the following: C2H4(g) + H2O(l) C2H5OH(l) • Tabulated G°f from Appendix 4: G°f(C2H5OH(l)) = -175 kJ/mol G°f(C2H4(g)) = 68 kJ/mol G°f(H2O (l)) = -237 kJ/mol • Using these values: C2H4(g) + H2O(l) C2H5OH(l) Grxn = Gprod. - Greact. G°rxn = G°f(C2H5OH(l)) - G°f(C2H4(g)) -G°f(H2O (l)) G°rxn = -175 kJ - 68 kJ -(-237 kJ) G°rxn = -6 kJ < 0 ; therefore, spontaneous More G° Calculations • Similar to H°, one can use the G° for various reactions to determine G° for the reaction of interest (a “Hess’ Law” for G°) • Example: C(s, diamond) + O2(g) CO2(g) G° = -397 kJ C(s, graphite) + O2(g) CO2(g) G° = -394 kJ C(s, diamond) + O2(g) CO2(g) G° = -397 kJ C(s, graphite) + O2(g) CO2(g) G° = -394 kJ CO2(g) C(s, graphite) + O2(g) G° = +394 kJ C(s, diamond) C(s, graphite) G° = -3 kJ G°rxn < 0…..rxn is spontaneous G°rxn ≠ Reaction Rate • Although G°rxn can be used to predict if a reaction will be spontaneous as written, it does not tell us how fast a reaction will proceed. • Example: C(s, diamond) + O2(g) CO2(g) G°rxn = -397 kJ <<0 But diamonds are forever…. G°rxn ≠ rate Example Problem • Is the following reaction spontaneous under standard conditions? 4KClO3 (s) 3KClO4 (s) KCl(s) H°f (kJ/mol) S° (J/mol.K) KClO3(s) -397.7 143.1 KClO4(s) -432.8 151.0 KCl (s) -436.7 82.6 Example Problem Solution • Calulating H°rxn 4KClO3 (s) 3KClO4 (s) KCl(s) Hrxn = 3H f (KClO4 ) H f (KCl) - 4H f (KClO3 ) = 3(-432.8kJ) (-436.7kJ) - 4(397.7kJ) = -144kJ • Calulating S°rxn Srxn = 3S(KClO4 ) S(KCl) - 4S(KClO3 ) = 3(151.0 J K ) (82.6 J K ) - 4(143.1 J K ) = -36.8 J K Example Problem Solution • Calulating G°rxn Grxn = Hrxn - TSrxn ( = -144kJ - (298K ) -38.6 J 1kJ K 1000J ) = -133kJ G°rxn < 0 ;therefore, reaction is spontaneous. Example Problem Continued For what temperatures will this reaction be spontaneous? Answer: For T in which Grxn < 0. Grxn = H rxn - TSrxn 0 = H rxn - TSrxn H rxn = Srxn -133kJ = 3446K = T 1kJ -38.6 J K 1000J ( ) Spontaneous as long as T < 3446 K.