Chapter 17
advertisement

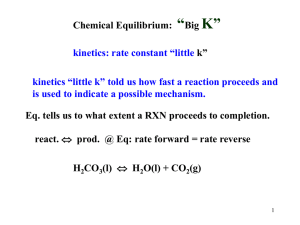
Chapter 17 1 FREE ENERGY AND THERMODYNAMICS SUROVIEC SPRING 2014 I. Spontaneous Change and Equilibrium 2 Chemical changes – reactions Physical changes – expansions, heat, precipitation Spontaneous change – occurs without outside intervention Does not tell us about rate of change Does indicate that we are headed to equilibrium II. Heat and Spontaneity 3 HCl (aq) + NaOH (aq) H2O (l) + NaCl (aq) DH = ? CH4 (g) + O2 (g) CO2 (g) + H2O (g) DH = ? NH4NO3 (s) NH4+ (aq) + NO3- (aq) DH = ? gas expanding : ? Melting of ice : DH = ? III. Dispersal of Energy 4 Entropy – S Second law of thermodynamics In a spontaneous process the entropy of the universe increases DS = S final - Sinitial A. Dispersal of Energy The dispersal of energy over as many different energy states is the KEY to entropy Thermal energy has been transferred 6 7 B. Dispersal of Matter 8 Since it is difficult to calculate to exact energy levels and dispersal of total energy among them lets initially look at the dispersal of matter among a system. B. Dispersal of Matter 9 Looking at the flask again, the entropy of a state increases with the number of energetically equivalent ways to arrange the components of the system. 2nd law states that for any spontaneous process, the entropy of the universe increases. C. Energy change and change in state 10 Entropy of a sample of matter changes as it changes state. IV. Heat transfer 11 There can also be spontaneous processes that occur even when entropy seems to decrease. B. Temperature dependence of DSsurr 12 If freezing increases entropy of surroundings, why would water not freeze at all temps? C. Quantifying entropy changes in the surrounding 13 When a system exchanges heat with the surroundings, it changes the entropy of the surroundings At constant pressure we can use qsys to quantify the change in entropy for the surroundings. C. Quantifying entropy changes in the surrounding 14 We also know that the higher the temperature the lower the magnitude of DSsurr Example 15 Consider the combustion reaction below: C3H8 (g) + 5O2(g) 3CO2(g) + 4H2O(l) ΔHrxn = -2044 kJ a)Calculate DSsurr at 25oC b)Determine the sign of DSsys c)Determine the sign of DSuniv Example 16 Given DH°rxn = -125kJ and ΔS°rxn = 253J/K at 25°C, what is the ΔSuniv? V. Gibbs Free Energy 17 Knowing that: DSuniv = DSsys - DH sys T We can use that to rearrange and solve for Gibbs free energy. V. Gibbs Free Energy 18 The change in Gibbs Free Energy for a process occurring at a constant temperature and pressure is proportional to the negative of ΔSuniv A. Effect of ΔH, ΔS and T 19 These terms are all related, but how? VI. So how do we know if a reaction is going to be spontaneous? 20 Example 21 Given: C2H4(g) + H2(g) C2H6(g) Where ΔH = -137.5kJ and ΔS = -120.5 J/K a)Calculate ΔG at 25°C b)How does T affect ΔG Example 22 Given: 2Ca(s) + o2(g) 2CaO(s) Where ΔH°rxn = -1269.8 kJ and ΔS°rxn = -364.6 J/K a)Calculate ΔG°rxn at 25°C b)Is it spontaneous? VI. Entropy changes in chemical reactions 23 As a reminder: ΔH°rxn is change in enthalpy for a process in which reactants and products are in their std. states. A. S° and the 3rd Law of Thermodynamics 24 Remember that we said that ΔH°f for an element in its std. state is zero. However since S is temperature dependent, we can better define the zero of entropy. B. Relative standard entropies 25 As we have seen, the entropy of a substance depends on its state. C. Calculating ΔS°rxn 26 To calculate ΔS°rxn we use the following equation: DS orxn = ånp S o (products) -ånr S o (reactants) Example 27 Compute ΔS°rxn for the following reaction: 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) J/molK NH3(g) 192.8 O2(g) 205.2 NO(g) 210.8 H2O(g) 188.8 Example 28 Compute ΔS°rxn for the following reaction: 2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g) J/molK H2S(g) 205.8 O2(g) 205.2 SO2(g) 248.2 H2O(g) 188.8 VI. Calculating ΔG°rxn 29 A. Free energy changes We can use ΔH°rxn and ΔS°rxn Example 30 Calculate the ΔG°rxn for the following reaction at 25°C 2SO2(g) + O2(g) 2SO3(g) ΔH°rxn ΔS°rxn kJ/mol J/molK SO2(g) -296.8 248.2 O2(g) 0 205.2 SO3(g) -395.7 256.8 Example 31 What is the previous reaction was not at 25°C but instead was at 125°C? B. ΔG°rxn using free energies 32 Since ΔG°rxn is the change in free energy and fee energy is a state function, we can solve ΔG°rxn as we did for ΔH°rxn and ΔS°rxn Example 33 Use standard free energies of formation to determine ΔG°rxn at 25°C CH4(g) + 8O2(g) CO2(g) + 2H2O(g) + 4O3(g) kJ/mol CH4(g) -50.5 O2(g) 0 CO2(g) -394.4 H2O(g) -228.6 O3(g) 163.2 C. Determining ΔG°rxn for a series of reactions 34 To determine ΔG°rxn for a series of reactions, we use the same rules that we did with ΔG°rxn Example 35 Determine the ΔG°rxn for the following reaction: 2NO(g) + O2(g) 2NO2(g) N2(g) + O2(g) 2NO(g) 2N2O(g) 2N2(g) + O2(g) ΔG°rxn = -71.2 kJ ΔG°rxn = +175.2 kJ ΔG°rxn = -207.4 kJ VII. DGo, K and product favorability 36 K = equilibrium constant How does K relate to ΔG°rxn ? ΔG°rxn is the increase or decrease in free energy as the reactants are completely converted to products Reactants are not always converted completely to products. VII. DGo, K and product favorability 37 When reactants are mixed, they proceed to position of lowest ree energy and then reach equilibrium. 38 Fig. 19-13, p.929 Combining Everything that we have learned 39 1. 2. 3. The free energy at equilibrium is lower than the free energy of reactants or products DGorxn gives the position of the equilibrium DGorxn describes the direction of the reaction 17_13.JPG