Crystallographic Point Groups
advertisement

Crystallographic Point Groups
Elizabeth Mojarro
Senior Colloquium
April 8, 2010
1
Outline
Group Theory
– Definitions
– Examples
Isometries
Lattices
Crystalline Restriction Theorem
Bravais Lattices
Point Groups
– Hexagonal Lattice Examples
We will be considering all of the above in R2 and R3
2
Groups Theory Definitions…
DEFINITION: Let G denote a non-empty set and let * denote a binary operation
closed on G. Then (G,*) forms a group if
(1) * is associative
(2) An identity element e exists in G
(3) Every element g has an inverse in G
Example 1: The integers under addition. The identity element is 0 and the
(additive) inverse of x is –x.
Example 2 : R-{0} under multiplication.
Example 3: Integers mod n. Zn = {0,1,2,…,n-1}.
If H is a subset of G, and a group in its own right, call H a subgroup of G.
3
Group Theory Definitions…
DEFINITION: Let X be a nonempty set. Then a bijection f: XX is called a
permutation. The set of all permutations forms a group under composition
called SX. These permutations are also called symmetries, and the group is
called the Symmetric Group on X.
DEFINITION: Let G be a group. If g G, then <g>={gn | n Z} is a
subgroup of G. G is called a cyclic group if g G with G=<g>. The element
g is called a generator of G.
Example: Integers mod n generated by 1. Zn= {0,1,2,…,n-1}.
All cyclic finite groups of n elements are the same (“isomorphic”) and are often
denoted by Cn={1,g,g2,…,gn-1} , of n elements.
4
Other Groups…
Example: The Klein Group (denoted V) is a 4-element group, which
classifies the symmetries of a rectangle.
5
More Groups…
DEFINITION: A dihedral group (Dn for n=2,3,…) is the group of symmetries
of a regular polygon of n-sides including both rotations and reflections.
n=3
n=4
6
The general dihedral group for a n-sided regular polygon is
Dn ={e,f, f2,…, fn-1,g,fg, f2g,…,fn-1g}, where gfi = f-i g, i. Dn is generated by the
two elements f and g , such that f is a rotation of 2π/n and g is the flip
(reflection) for a total of 2n elements.
f
7
Isometries in R2
DEFINITION: An isometry is a permutation : R2 R2 which preserves
Euclidean distance: the distance between the points of u and v equals the
distance between of (u) and (v). Points that are close together remain close
together after .
8
Isometries in R2
The isometries in are Reflections, Rotations, Translations, and Glide Reflections.
9
Invariance
Lemma: The set of all isometries that leave an object invariant form a group under
composition.
Proof: Let L denote a set of all isometries that map an object BB.
The composition of two bijections is a bijection and composition is associative.
Let α,β L.
αβ(B)= α(β(B))
= α(B) Since β(B)=B
=B
Identity: The identity isometry I satisfies I(B)=B and Iα= αI= α for α L.
Inverse: 1 ( B) 1 ( ( B)) ( 1 )(B) B
Moreover the composition of two isometries will preserve distance.
10
Crystal Groups in R2
DEFINITION: A crystallography group (or space group) is a group of
isometries that map R2 to itself.
DEFINITION: If an isometry leaves at least one point fixed then it is a point
isometry.
DEFINITION: A crystallographic group G whose isometries leave a common
point fixed is called a crystallographic point group.
Example: D4
11
Lattices in R2
Two non-collinear vectors a, b of minimal length form a unit cell.
DEFINITION: If vectors a, b is a set of two non-collinear nonzero vectors in
R2, then the integral linear combinations of these vectors (points) is called a
lattice.
Unit Cell:
Lattice :
12
Lattice + Unit Cell
Crystal in R2 superimposed on a lattice.
13
Crystalline Restriction Theorem in R2
What are the possible rotations around a fixed point?
THEOREM: The only possible rotational symmetries of a lattice are 2-fold, 3-fold,
4-fold, and 6-fold rotations (i.e. 2π/n where n = 1,2,3,4 or 6).
14
Crystalline Restriction Theorem in R2
Proof: Let A and B be two distinct points at minimal distance.
Rotate A by an angle α , yielding A’
Rotating B by - α yields
Together the two rotations yield:
A’
B’
|r ’|
|r|
|r|
α
-α
A
|r|
B
15
Possible rotations:
Case 1: |r'|=0
Case 2: |r'| = |r|
α= π/3 = 2π/6
Case 3 : |r'| = 2|r|
α= π/2 = 2π/4
Case 4: |r'| = 3|r|
|r|
|r|
|r|
α= π = 2π/2
α= 2π/3
16
Bravais Lattices in R2
Given the Crystalline Restriction Theorem, Bravais Lattices are the only
lattices preserved by translations, and the allowable rotational symmetry.
17
Bravais Lattices in R2 (two vectors of equal length)
Case 1:
Case 2:
18
Bravais Lattices in R2 (two vectors of unequal length)
Case 1:
Case 2:
Case 3:
19
Point Groups in R2 – Some Examples
Three examples
Point groups:
C2, C4 , D4
Point groups:
C2, D3 , D6, C3 , C6 , V
20
C3
21
Isometries in R3 (see handout)
Rotations
Reflections
Improper Rotations
Inverse Operations
22
Lattices in R3
Three non-coplanar vectors a, b, c of minimal length form a unit cell.
DEFINITION: The integral combinations of three non-zero, non-coplanar
vectors (points) is called a space lattice.
Unit Cell:
Lattice:
23
Bravais Lattices in R3
The Crystalline Restriction Theorem in R3 yields
14 BRAVAIS LATTICES in
7 CRYSTAL SYSTEMS
Described by “centerings” on different “facings” of the unit cell
24
The Seven Crystal Systems Yielding 14 Bravais Latttices
Triclinic:
Tetragonal:
Monoclinic:
Orthorhombic:
Trigonal:
25
Hexagonal:
Cubic:
26
Crystallography Groups and Point Groups in R3
Crystallography group (space group)
(Crystallographic) point group
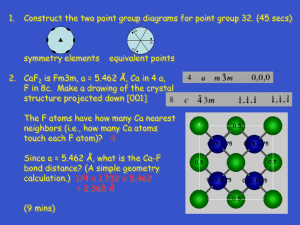
32 Total Point Groups in R3 for the 7 Crystal Systems
27
Table of Point Groups in R3
Crystal
system/Lattice
system
Point Groups
(3-D)
Triclinic
C1, (Ci )
Monoclinic
C2, Cs, C2h
Orthorhombic
D2 , C2v, D2h
Tetragonal
C4, S4, C4h, D4 C4v,
D2d, D4h
Trigonal
C3, S6 (C3i), D3 C3v,
D3d
Hexagonal
C6, C3h, C6h, D6
C6v, D3h, D6h
Cubic
T, Th ,O ,Td ,Oh
28
The Hexagonal Lattice
29
{1,6}{6,5}
30
{1,6}{5,4}
{5,4}{12,11}
31
{1,6}{6,5}
{6,5}{13,12}
32
{1,6}{6,5}
{6,5}{13,8}
33
{1,6}{5,4}
{5,4}{8,9}
{8,9}{1,2}
34
{1,6}{6,5}
{6,5}{8,13}
{8,13}{6,1}
35
{1,6} {6,5}
{6,5}{2,3}
36
Boron Nitride (BN)
37
Main References
Boisen, M.B. Jr., Gibbs, G.V., (1985). Mathematical Crystallography: An
Introduction to the Mathematical Foundations of Crystallography. Washington,
D.C.: Bookcrafters, Inc.
Crystal System. Wikipedia. Retrieved (2009 November 25) from
http://en.wikipedia.org/wiki/Crystal_system
Evans, J. W., Davies, G. M. (1924). Elementary Crystallography. London: The
Woodbridge Press, LTD.
Rousseau, J.-J. (1998). Basic Crystallography. New York: John Wiley & Sons,
Inc.
Sands, D. E (1993). Introduction to Crystallography. New York: Dover
Publication, Inc.
Saracino, D. (1992). Abstract Algebra: A First Course. Prospect Heights, IL:
Waverland Press, Inc.
38
Special Thank You
Prof. Tinberg
Prof. Buckmire
Prof. Sundberg
Prof. Tollisen
Math Department
Family and Friends
39