File
advertisement

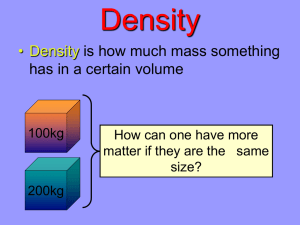
Worksheet # 3 GRADED Problem 1 &2 If the mass of 50 mL of gasoline is 34 g, 1. What is its density? 2. Will gasoline float or sink if dropped in a basin full of water? (Note: Density of Water = 1 g/mL) Problem 3 3. Mercury has a density of 13.55 g/cm3. If 250 mL of mercury is to be used in constructing a mercury thermometer. What would be the mass of the mercury? Problem 4. 4. An irregularly-shaped sample of aluminum (Al) is put on a balance and found to have a mass of 43.6 g. The student decides to use the water-displacement method to find the volume. The initial volume reading is 25.5 mL and, after the Al sample is added, the water level has risen to 41.7 mL. Find the density of the Al sample in g/cm3. (Remember: 1 mL = 1 cm3.) Problem 5,6, & 7 30.0 g of each of the following acids are needed. Find the volume of each that must be measured out in a graduated cylinder. 5. hydrochloric acid (HCl), density = 1.164 g/mL 6. sulfuric acid (H2SO4), density = 1.834 g/mL 7. nitric acid (HNO3), density = 1.251 g/mL PROBLEM 8, 9, 10 Promethium has a density of 7.26 grams/cm3 and Europium has a density of 5.24 g/cm3. 8. Which would have the greater mass, 12 cm3 of Promethium or 17 cm3 of Europium? 9 & 10 ENCIRCLE THE MASS OF BOTH METALS. DENSITY OF WATER = 1 g/mL SINK/FLOA DENSITY T FORMULA MASS VOLUME 11 45 grams 30 mL 12 13 14 15 8 mL 0.45 g/mL 16 17 100 grams 18 2.5 g/mL 19 20 84 grams 112 mL 21 22 23 24 20 mL 0.99 g/mL 25 Problem 1 &2 If the mass of 50 mL of gasoline is 34 g, 1. What is its density? 0.68 g/mL D= M/V= 34g / 50 mL= 0.68 g/mL 2. Will gasoline float or sink if dropped in a basin full of water? Float because if the density is lower it will float. (Note: Density of Water = 1 g/mL) Problem 3 3. Mercury has a density of 13.55 g/cm3. If 250 mL of mercury is to be used in constructing a mercury thermometer. What would be the mass of the mercury? 3,387.5 grams M= D x V= 13.55 g/cm3 x 250 mL= 3,387.5 g Problem 4. 4. An irregularly-shaped sample of aluminum (Al) is put on a balance and found to have a mass of 43.6 g. The student decides to use the water-displacement method to find the volume. The initial volume reading is 25.5 mL and, after the Al sample is added, the water level has risen to 41.7 mL. Find the density of the Al sample in g/cm3. (Remember: 1 mL = 1 cm3.) 2.69 g/mL Volume= 41.7 mL - 25.5 mL= 16.2 mL D= M / V= 43.6 g / 16.2 mL= 2.69 g/mL Problem 5,6, & 7 30.0 g of each of the following acids are needed. Find the volume of each that must be measured out in a graduated cylinder. 5. hydrochloric acid (HCl), density = 1.164 g/mL V= M / D= 30 g/ 1.164 g/mL= 25.77 mL 6. sulfuric acid (H2SO4), density = 1.834 g/mL V= M / D= 30 g/ 1.834 g/mL= 16.36 mL 7. nitric acid (HNO3), density = 1.251 g/mL V= M / D= 30 g/ 1.251 g/mL= 23.98 mL PROBLEM 8, 9, 10 Promethium has a density of 7.26 grams/cm3 and Europium has a density of 5.24 g/cm3. 8. Which would have the greater mass, 12 cm3 of Promethium or 17 cm3 of Europium? 9 & 10 ENCIRCLE THE MASS OF BOTH METALS. Promethium= M= D x V= 7.26 x 12= 87.12 g Europium= M= D x V= 5.24 x 17= 89.08 g Europium has a bigger mass. DENSITY OF WATER = 1 g/mL SINK/FLOA DENSITY T FORMULA MASS VOLUME d= m/v 45 grams 30 mL 1.5 g/mL sink m= d x v 3.6 grams 8 mL 0.45 g/mL float v= m/d 100 grams 40 mL 2.5 g/mL sink d= m/v 84 grams 112 mL 0.75 g/mL float m= d x v 19.8 grams 20 mL 0.99 g/mL float