GRAPHING AND VARIABLES
advertisement
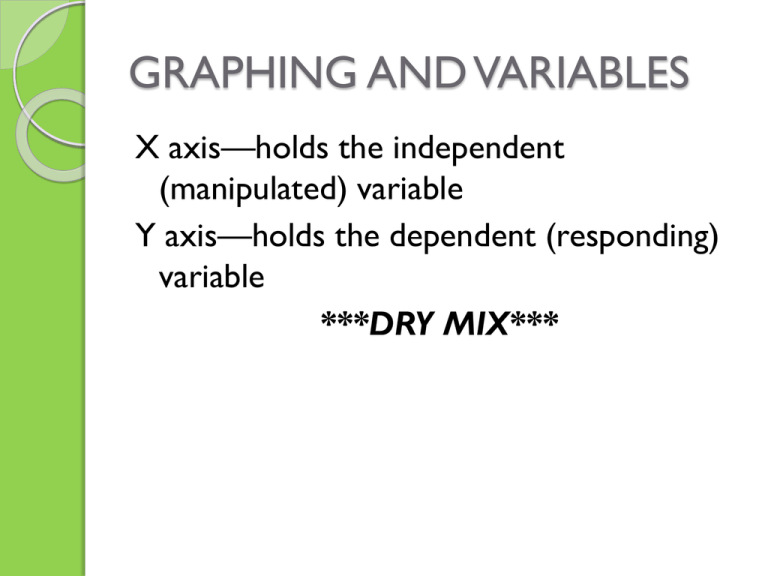
GRAPHING AND VARIABLES X axis—holds the independent (manipulated) variable Y axis—holds the dependent (responding) variable ***DRY MIX*** ACTIVATOR: WORD SPLASH--MEASUREMENT List as many terms or phrases that we’ve discussed in unit 2 that deal with measurement DATA TABLES AND VARIABLES X (INDEPENDENT) Y (DEPENDENT) Ex: Volume (mL) Ex: Mass (grams) 2.0 7.9 3.0 16.2 4.0 25.0 5.0 32.3 SLOPE Is determined by the first and last data points Last y – first y = y2 - y1 last x – first x x2 - x1 Ex: 32.3 – 7.9 5.0 – 2.0 = 24.4 = 8.1 3.0 DIRECT VS. INDIRECT PROPORTIONS DIRECT INDIRECT (aka INVERSE) Shows a negative slope Factors do opposite things Shows a positive slope both factors increase or both factors decrease (do the same thing) Ex: speed = distance/time Ex: area = length x width density = mass/ volume Graph: Graph: FLIPPED ASSIGNMENT--DENSITY Visit the following website: http://mswipc.com/problem_sets/density/de nsity_sample_problems.htm Review all 3 types of density problems posted on this page before class tomorrow. ACTIVATOR: Explain why these substances are layered? DENSITY DEMONSTRATIONS: (TEACHERTUBE.COM) 1. The density of liquids 2. Underwater volcano 3. The density of water is 1 4. Dr. Reich’s density lab http://www.teachertube.com/viewVideo.ph p?video_id=7593 FOR DENSITY LAB: --follow your procedure !!! ---DO NOT MOVE CHAIRS AROUND ---MUST KEEP GOGGLES ON AT ALL TIMES at station 2!!! ---PART OF YOUR GRADE IS BASED ON CLEAN UP ---WHEN USING SOAP TO WASH CYLINDER, ONLY USE 1 DROP! ---stay with your group and stay quiet!! --- calculate all densities after coming to sit down On your data table, add: PART C: Mass of red block= Length = Width= Height= Volume = Density = DENSITY LAB CONCLUSIONS ANSWER 1—5 For #2 you will need these accepted values. % error = measured – accepted x 100 accepted Water = 1.00 g/mL Liquid A (isopropanol)= 0.786 g/mL Liquid B (glycerin) = 1.26 g/mL Solid sinker (lead alloy) = 11.40 g/cc Red wood (alder) block = 0.4 g/cc DENSITY DENSITY = MASS VOLUME **this is a direct proportion** As mass increases, volume increases (or as mass decreases, volume decreases) Units that measure density: Solids: g/cm3 liquids: g/mL gases: g/L **see density chart p. 38 Specific gravity The density of a substance compared to water’s density (1.0 g/mL) Float = density less than water Sink = density greater than water p. 40 (SHOW WORK AND INCLUDE CORRECT UNIT) #1. What is the density of a block of marble occupying 310. Cm3 and a mass of 853 grams? #2. Diamond has a density of 3.26 g/cm3 and a volume of 0.351 cm3. Find the mass. #3. What is the volume of a sample of mercury having a mass of 76.2 grams and a density of 13.6 g/mL? ANSWERS 1. 2.75 g/cm3 2. 1.14 grams 3. 5.60 mL 4) If a sample of lead is cut in half, will its density change? Explain why or why not. No b/c mass AND volume both change by the same factor 5) Find the density in grams/mL of a liquid having a mass of 2 kg and a volume of 2900 mL. What might this liquid be? (see p. 38) Would it sink or float in water? 2000 grams / 2900 mL = 0.7 g/mL It would float because it’s density is less than water’s SUMMARIZER: DENSITY 3-2-1 Name 3 measurements the density formula involves. Give 2 units that measure density. Give 1 reason an object may float in water. ACTIVATOR: (using your calculator) Calculate the density of a gas with a mass of 253 grams and a volume of 3.5 L. SHOW YOUR WORK ACTIVATOR: Using the Density chart below, name 2 substances that will float in water AND 2 substances that will sink in water. Brass 8.55 Gold 19.3 Iron 7.8 Uranium 18.7 Ice at 0 C 0.92 Wood 0.67 Homework Density handout front 1—13 back ODD ****also read LAB carefully Finish IPAD activity DENSITY HANDOUT ANSWERSFRONT SIDE 1. 1.20 g/mL 2. 3.3 g/cc (could be Aluminum which has density of 2.7 g/cc) 3. 700 grams 4. 200 mL 5. 0.757 g/cc 6. 73 mL 7. 1.12 g/mL 8. 290 grams 9. 70 cc 10. 1.53 g/mL 11. 1.8 g/cc 12. 600 grams 13. 1.44 g/mL BACK---ODD 1--20 1. 3. 5. 7. 9. 11. 13. 15. 17. 19. 0.23 g/cc 0.14 g/cc 0.21 g/cc 0.27 g/cc 0.11 g/cc 2.0 g 2.7 g 2.6 g 90. cc 41 cc EXTRA PRACTICE --DENSITY 1. 2. 3. 4. 5.. 30 2.0 2 3.0 SUMMARIZER—using the density formula How is density calculated? How is mass calculated? How is volume calculated? ACTIVATOR Calculate the volume of a block of wood having a denisty of 0.78 g/cm3 and a mass of 300 grams. WARM UP—DENSITY & GRAPHING 1. Calculate the density of a liquid having a mass of 35 grams and a volume of 111 mL 2. Calculate the mass of a solid having a density of 2.5 g/cc and a volume of 3 cc. 3. Calculate the volume of a block with a mass of 9.0 grams and a density of 45 g/cm3. 4. Find the density in grams/mL of a liquid having a mass of 3.4 kg and a volume of 1.0 L. 5. If a sample of gold is cut in half, would its density change. EXPLAIN WHY OR WHY NOT. ANSWER THE FOLLOWING QUESTIONS USING THE DATA TABLE BELOW: MASS (g) VOLUME (mL) 3.0 15.0 4.0 10.0 5.0 6.0 6. 7. 8. 9. Calculate slope of the graph’s line. Is this a direct or indirect relationship? What is the responding variable? What is the independent variable? SUMMARIZER Describe how the density is measured for an irregular shaped solid VS. a rectangular shaped solid. p. 42 #5 p. 54 #1, 2 p. 57 #7-10 p. 59 # 13b,c 14a,b 17-19, 22a,b 32b, 33b ACTIVATOR Put the object’s in the lab in order of least to greatest density: Water = 1.00 g/mL Liquid A (isopropanol)= 0.786 g/mL Liquid B (glycerin) = 1.26 g/mL Solid sinker (lead alloy) = 11.40 g/cc Red wood (alder) block = 0.4 g/cc Which ones would sink in water? Which ones would float in water? QUIZ-- DENSITY= mass/volume REMEMBER SIG FIGS AND UNITS!! SHOW WORK!! 1. Calculate the density of a solid having a mass of 45 grams, a length of 2 cm, a width of 3 cm, and a height of 1 cm. 2. An object’s density is 3.6 g/cc. It is put into a graduated cylinder initially having 25.0 mL. The water level rises to 25.2 mL. What is the object’s mass? 3. Find the volume of an object with a mass of 25 kg and a density of 5 g/mL.











