Density= Mass
advertisement

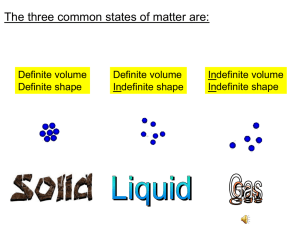
DENSITY Review DENSITY_COMPUTATION slide found on the SCIENCE weebly site. Graded Worksheet tom. DENSITY = MASS / VOLUME DENSITY= grams/ mL or kg/L VOLUME= MASS / DENSITY MASS= DENSITY X VOLUME Note: Higher density sinks/ stays at the bottom and lower density stays on top/ floats. Lowest Density = Lamp Oil Highest Density = Honey The substance that stays on top has the lowest density and he substance that stays at the bottom has the highest dens PROBLEM I 1. What is the density of CO gas if 0.196 g occupies a volume of 100 ml? PROBLEM II 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury. PROBLEM III 3. An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. The height of the water rose to 7 ml. If the mass of the stone was 25 g, what was its density? PROBLEM IV 4. Silver has a density of 10.5 grams/cm3 and gold has a density of 19.3 g/cm3. Which would have the greater mass, 5cm3 of silver or 5cm3 of gold? PROBLEM V 5. Five mL of ethanol has a mass of 3.9 g, and 5.0 mL of benzene has a mass of 44 g. Which liquid is denser? DENSITY OF WATER = 1 g/mL FORMULA MASS VOLUME DENSITY SINK/FLOAT ? 30 grams 80 mL ? ? 1 II 6 mL 2.5 g/mL III IV 50 grams V 0.5 g/mL VI VII 77 grams 140 mL VIII IX X XI 38 mL 4.5 g/mL XII PROBLEM I 1. What is the density of CO gas if 0.196 g occupies a volume of 100 ml? Density= Mass/ Volume Density= 0.196g / 100 mL Density= 0.00196 g/ mL PROBLEM II 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury. Density= Mass / Volume Density= 306 g/ 22.5 mL Density= 13.6 g/ mL PROBLEM III 3. An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. The height of the water rose to 7 ml. If the mass of the stone was 25 g, what was its density? Density= Mass / Volume Density= 25 g/ (7 mL - 2 mL) = 25 g/ 5 mL Density= 5 g/ mL PROBLEM IV 4. Silver has a density of 10.5 grams/cm3 and gold has a density of 19.3 g/cm3. Which would have the greater mass, 5cm3 of silver or 5cm3 of gold? Mass= Density x Volume Mass (silver)= 10.5 g/cm3 x 5 cm3= 52.5 cm3 Mass (gold)= 19.3 g/ cm3 x 5 cm3= 96.5 cm3 (greater) PROBLEM V 5. Five mL of ethanol has a mass of 3.9 g, and 5.0 mL of benzene has a mass of 44 g. Which liquid is denser? Density= Mass / Volume Density ethanol = 3.9 g/ 5 mL= 0.78 g/mL Density benzene = 44 g/ 5 mL= 8.8 g/mL Benzene is denser than ethanol. DENSITY OF WATER = 1 g/mL FORMULA MASS VOLUME DENSITY SINK/FLOA T D=M/V 30 grams 80 mL 0.375 g/mL float M=D x V 15 grams 6 mL 2.5 g/mL sink V= M/D 50 grams 100 mL 0.5 g/mL float D=M/V 77 grams 140 mL 0.55 g/mL float M=D x V 171 grams 38 mL 4.5 g/mL sink