Introduction to Crystallography
advertisement

Practical I - A
INTRODUCTION TO
CRYSTALLOGRAPHY
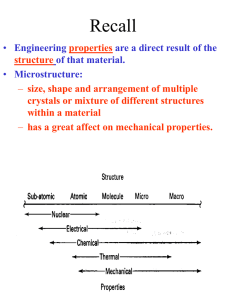
The crystal lattice
The unit cell
Unit cell – polyhedra with 3 pairs of parallel faces (parallelepiped) that repeat
periodically in 3 dimensions
General rules for choosing the unit cell:
Must have an integral number of formula units (eg.: halite:
1 Na, 1 Cl)
Each corner must be identical (eg.: halite: Cl must occupy
all corners)
Express symmetry of the atomic relationship
Choosing the
unit cell
14 Bravais lattices
Triclinic
Monoclinic
Orthorhombic
Tetragonal
(NB: Trigonal = rhombohedral)
Trigonal
(Rhombohedral)
Hexagonal
Cubic (Isometric)
Crystallographic axis
One of three lines (sometimes four, in the case of a hexagonal
crystal), passing through a common point, that are chosen to
have definite relation to the symmetry properties of a crystal,
and are used as a reference in describing crystal symmetry and
structure.
The crystallographic axes are imaginary lines that we can draw within
the crystal lattice.
These will define a coordinate system within the crystal.
For 3-dimensional space lattices we need 3 or in some cases 4
crystallographic axes that define directions within the crystal lattices.
Depending on the symmetry of the lattice, the directions may or may
not be perpendicular to one another, and the divisions along the
coordinate axes may or may not be equal along the axes.
The lengths of the axes are in some way proportional to the lattice
spacing along an axis and this is defined by the unit cell.
Crystal symmetry
Symmetrically arranged faces reflect the internal arrangement of
atoms. The symmetry can be described according to three symmetry
elements:
Centre of symmetry
A central point which is present when all faces or edges occur in parallel pairs on
opposite sides of the crystal.
A point, within a crystal, through which any straight line also passes through two
points on the edge of the figure at the same distance from the centre but on
opposite sides.
The centre of symmetry at a point (0,0,0) operates on any point (x,y,z) to give an
identical point at (-x,-y,-z).
Axis of symmetry
A line about which a crystal may be rotated through 360°/n until it assumes a
congruent position (identical image is seen); n may equal 2, 3, 4 or 6 – depending on
the number of times the congruent position is repeated, resulting in 2-fold (diad), 3fold (triad), 4-fold (tetrad) and 6-fold (hexad) axes.
Plane of symmetry (also mirror plane)
A plane by which the crystal may be divided into two halves which are mirror images
of each other.
Videos
Crystallographic classification system
Using the elements of symmetry discussed
above, crystallographers have recognized
32 Crystal classes (point groups)
Classified based on three symmetry operations
6(7) Crystal systems
Classified based on lattice parameters (a, b, c and α, β, γ)
Symmetry is highest (high symmetry) in the cubic
system, where many elements are repeated, and
lowest (low symmetry) in the triclinic system, where
only a centre of symmetry may be present (i.e. there
may be no plane or axis of symmetry).
Crystal forms (230 space groups)
All known crystal forms fit into the above
seven crystal systems. But why don't all
crystals in a given set look the same?
Or, stated differently, why can't I learn seven
crystal shapes and know all I need to know?
Well, crystals, even of the same mineral, have
differing CRYSTAL FORMS, depending upon
their conditions of growth.
Whether they grew rapidly or slowly, under
constant or fluctuating conditions of
temperature and pressure, or from highly
variable or remarkably uniform fluids or melts,
all these factors have their influence on the
resultant crystal shapes, even when not
considering other controls.
Video
Practical
Classify your own examples
Concept of a lattice and
description of crystal
structures
Sources:
HR Wenk and A Bulakh, 2004, Minerals: Their
Constitution and Origin, Univ Press, Cambridge
© DoITPoMS, University of Cambridge
http://www.doitpoms.ac.uk/tlplib/miller_indices
Introduction
Miller Indexing is a method of describing the orientation of
a plane or set of planes within a lattice in relation to the unit
cell.
Miller Indices were developed by William Hallowes Miller.
These indices are useful in understanding many
phenomena in materials science, such as explaining the
shapes of single crystals, the form of some materials'
microstructure, the interpretation of X-ray diffraction
patterns, and the movement of a dislocation , which may
determine the mechanical properties of the material.
How to index a lattice
How to index a lattice
How to index a plane
How to index a plane
How to index a plane
The zero index
Negative indices
Parallel planes
Lattice planes can be
represented by
showing the trace of
the planes on the
faces of one or more
unit cells. The
diagram shows the
trace of the () planes
on a cubic unit cell.
Bracket Conventions
In crystallography there are conventions as to how the indices of planes and
directions are written. When referring to a specific plane, “round” brackets are
used:
When referring to a set of planes related by symmetry, then “curly” brackets are
used:
{hkl}
These might be the (100) type planes in a cubic system, which are (100), (010),
(001), (ī00) (0ī0) and (00ī). These planes all “look” the same and are related to
each other by the symmetry elements present in a cube, hence their different
indices depend only on the way the unit cell axes are defined. That is why it
useful to consider the equivalent (010) set of planes
Directions in the crystal can be labeled in a similar way. These are effectively
vectors written in terms of multiples of the lattice vectors a, b, and c. They are
written with “square” brackets:
(hkl)
[UVW]
A number of crystallographic directions can also be symmetrically equivalent, in
which case a set of directions are written with “triangular” brackets:
<UVW>
Examples of lattice planes
The (100), (010), (001), (ī00), (0ī0) and (00ī) planes form the faces
of the unit cell.
Here, they are shown as the faces of a triclinic (a ≠ b ≠ c, α ≠ β ≠ γ)
unit cell . Although in this image, the (100) and (ī00) planes are
shown as the front and back of the unit cell, both indices refer to
the same family of planes.
It should be noted that these six planes are not all symmetrically
related, as when they are in the cubic system
Examples of lattice planes
The (101), (110), (011), (10ī), (1ī0) and (01ī) planes
form the sections through the diagonals of the unit
cell, along with those planes whose indices are the
negative of these, eg.: (ī0ī); (ī01); (ī10); (0ī1), .
In the image the planes are shown in a different
triclinic unit cell.
Practical work
Q1: Determine the Miller Indices
for plane A and B
Practical assignment
Q2: Construct the faces indicated here on the
different faces and assign Miller indices to
each one (A to L)
Draw your own lattice planes
The following link shows you simulations
generating images of lattice planes as you
enter a set of Miller indices (each index
between 6 and -6) in the following format:
(1;-2;0)
http://www.doitpoms.ac.uk/tlplib/miller_indic
es/lattice_draw.php
ANSWERS
Answers:
Q1:
A: 112
B: 221
Answers
Q2:
Worked examples
The figure below is a scanning electron micrograph of a niobium carbide
dendrite in a Fe-34wt%Cr-5wt%Nb-4.5wt%C alloy.
Niobium carbide has a face centred cubic lattice.
The specimen has been deep-etched to remove the surrounding matrix
chemically and reveal the dendrite.
The dendrite has 3 sets of “arms”
which are orthogonal to one another
(one set pointing out of the plane of
the image, the other two sets, to a
good approximation, lying in the plane
of the image), and each arm has a
pyramidal shape at its end.
It is known that the crystallographic
directions along the dendrite arms
correspond to the < 100 > lattice
directions, and that the
direction ab labelled on the
micrograph is [10ī]