3. - Aamir Razaq
advertisement

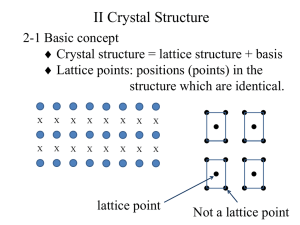
Wigner-Seitz Cell
The Wigner–Seitz cell around a lattice point is defined as the locus
of points in space that are closer to that lattice point than to any of
the other lattice points.
Wigner-Seitz Method
A simply way to find the primitive
cell which is called Wigner-Seitz
cell can be done as follows;
1. Choose a lattice point.
2. Draw lines to connect these
lattice point to its neighbours.
3. At the mid-point and normal
to these lines draw new
lines.
The volume enclosed is called as a
Wigner-Seitz cell.
Crystal Structure
2
Square lattice
Centred Rectangular lattice
Wigner-Seitz cells
Different kinds of CELLS
Unit cell
A unit cell is a spatial arrangement of atoms which is tiled in three-dimensional
space to describe the crystal.
Primitive unit cell
For each crystal structure there is a conventional unit cell, usually chosen to make
the resulting lattice as symmetric as possible. However, the conventional unit cell is
not always the smallest possible choice. A primitive unit cell of a particular crystal
structure is the smallest possible unit cell one can construct such that, when tiled, it
completely fills space.
Wigner-Seitz cell
A Wigner-Seitz cell is a particular kind of primitive cell
which has the same symmetry as the lattice.
Wigner-Seitz Cell - 3D
Crystal Structure
5
Lattice Sites in Cubic Unit Cell
Crystal Structure
6
Lecture 03
CRYSTALLOGRAPHIC POINTS, DIRECTIONS,
PLANES
&
THE MILLER SYSTEM OF INDICES
Fundamental properties of Solids
Crystal Directions
•
•
We choose one lattice point on the line
as an origin, say the point O. Choice of
origin is completely arbitrary, since
every lattice point is identical.
Then we choose the lattice vector
joining O to any point on the line, say
point T. This vector can be written as;
R = n1 a + n2 b + n3c
•
•
To distinguish a lattice direction from a
lattice point, the triple is enclosed in
square brackets [ ...] is used.[n1n2n3]
[n1n2n3] is the smallest integer of the
same relative ratios.
Crystal Structure
Fig. Shows
[111] direction
8
Examples
210
X=½ ,Y=½,Z=1
[½ ½ 1]
[1 1 2]
X=1,Y=½,Z=0
[1 ½ 0]
[2 1 0]
Crystal Structure
9
Negative directions
• When we write the
direction
[n1n2n3]
depend on the origin,
negative
directions
can be written as
Z direction
[n1n2n3 ]
• R = n1 a + n2 b + n3c
(origin) O
- X direction
- Y direction
Y direction
[n1n2n3 ]
Direction must be
smallest integers.
X direction
- Z direction
Crystal Structure
10
Examples of crystal directions
X=1,Y=0,Z=0
[1 0 0]
X = -1 , Y = -1 , Z = 0
Crystal Structure
[110]
11
Examples
We can move vector to the origin.
X =-1 , Y = 1 , Z = -1/6
[-1 1 -1/6]
[6 6 1]
Crystal Structure
12
When dealing with crsytalline materials, it is often
necessary to specify a particular point within a unit
cell, a particular direction or a particular plane of
atoms.
Planes are important in crystals because if bonding
is weak between a set of parallel planes, then brittle
shear fracture may occur along these planes.
Therefore, it is necessary to be able to specify
individual crystal planes and in the case of shear to
specify directions within these planes.
Such identification is carried out by means of
Miller Indices.
CRYSTALLOGRAPHIC DIRECTIONS
A crystallographic direction is defined as a line
between two points (a vector).
1. A vector of convenient length is positioned such that it passes
through the origin of the coordinate system. (Any vector can be
translated throughout the crystal lattice, if parallelism is
maintained).
2. The length of the vector projection on each of the three axes is
determined in terms of the unit cell dimensions a, b, and c.
3. These three numbers are multiplied or divided by a common
factor to reduce them to the smallest integer values.
4. The tree indices are enclosed in brackets as [uvw]. The u, v,
and w integers correspond to the reduced projections along x,
y, and z-axes respectively.
z
C
A
a
x
B
y
a
a
• Vector A → a, a, a 1/a, 1/a, 1/a
• Vector B → [1 1 0]
• Vector C → [1 1 1]
[1 1 1]
• For some crystal structures, several nonparallel directions with
different indices are actually equivalent. (The spacing of atoms
along each direction is the same)
• For example in cubic crystals, all the directions represented
by the following indices are equivalent.
[100],[1 00],[010],[0 1 0],[001],[001]
• As a convenience, equivalent directions are
grouped into a “family” which are grouped in angle
brackets.
[
100
],
[
1
00
],
[
010
],
[
0
1
0
],
[
001
],
[
00
1
]
100
Sometimes the angle between two directions
may be necessary.
A [h1 k1 l1] and B [h2 k2 l2] → the angle
between them is a.
A . B=|A| |B| cos a
cos a =
h1h2 + k1k2 + l1l2
(h12+k12+l12) (h22+k22+l22)
Crystal Planes
•
•
Within a crystal lattice it is possible to identify sets of equally
spaced parallel planes. These are called lattice planes.
In the figure density of lattice points on each plane of a set is
the same and all lattice points are contained on each set of
planes.
The set of
planes in
2D lattice.
b
b
a
a
Crystal Structure
18
CRYSTALLOGRAPHIC PLANES
• The orientations of planes for a crystal
structure are represented in a similar manner.
• In all but the hexagonal crystal system,
crystallographic planes are specified by three
Miller Indices as (hkl).
• Any two parallel planes are equivalent and
have identical indices.
• The following procedure is employed in the
determining the h, k, and l idex numbers of a
plane:
1.
If the plane passes through the selected origin, either another
parallel plane must be constructed within the unit cell by an
appropriate translation, or a new origin must be established at
the corner of another unit cell.
2. At this point the crystallographic plane either intersects or
parallels each of the three axes; the length of the planar
intercept for each axis is determined in terms of the lattice
parameters a, b, and c.
3. The reciprocals of these numbers are taken.
4. If necessary, these three numbers are changed to the set of
smallest integers by multiplication or division by a common
factor.
5. The integer indices are enclosed within parantheses as (hkl).
1. The plane passes
through the
selected origin O.
Therefore, a new
origin must be
selected at the
corner of an
adjacent unit cell.
Various non-parallel planes may have similarities
(crystallographically equivalent ). Such planes are
referred to as “family of planes” and are designated
as {h k l}
Example: Faces of a cubic unit cell.
(100)
(010)
(001)
(100)
(010)
(001)
Ξ {100}
Indices of Planes: Cubic Crystal
LINEAR DENSITY
When planes slip over each other, slip
takes place in the direction of closest
packing of atoms on the planes.
The linear density of a crystal direction [h k l]
is determined as:
δ[h k l] =
# of atoms
length
FCC: Linear Density
• Linear Density of Atoms LD =
Number of atoms
Unit length of direction vector
[110]
ex: linear density of Al in [110]
direction
a = 0.405 nm
# atoms
a
Adapted from
Fig. 3.1(a),
Callister &
Rethwisch 8e.
LD =
length
2
= 3.5 nm-1
2a
26
BCC: Linear Density
Calculate the linear density for the
following directions in terms of R:
a. [100]
b. [110]
c. [111]
Planar Density of (100) Iron
Solution: At T < 912ºC iron has the BCC structure.
2D repeat unit
(100)
Planar Density =
area
2D repeat unit
1
a2
=
4 3
R
3
Radius of iron R = 0.1241 nm
Adapted from Fig. 3.2(c), Callister & Rethwisch 8e.
atoms
2D repeat unit
a=
1
4 3
R
3
atoms
atoms
19
= 1.2 x 10
2 = 12.1
2
nm
m2
28
P 3.55 (a): Planar Density for
BCC
Derive the planar density expressions for BCC (100) and
(110) planes in terms of the atomic radius R.
Planar Density of BCC (111)
Iron1 atom in plane/ unit surface cell
Solution (cont): (111) plane
2a
atoms in plane
atoms above plane
atoms below plane
h=
3
a
2
2
atoms
2D repeat unit
4 3 16 3 2
2
area = 2 ah = 3 a = 3
R =
R
3
3
1
atoms =
= 7.0
2
Planar Density =
area
2D repeat unit
16 3
3
R
2
nm
0.70 x 1019
atoms
m2
30
P 3.54 (a): FCC
Derive planar density expressions for FCC (100), (110),
and (111) planes.
P 3.56
3.56 (a) Derive the planar density expression for the HCP
(0001) plane in terms of the atomic radius R.
(b) Compute the planar density value for this same
plane for magnesium. (atomic radius for magnesium is
0.160 nm)
32