点击下载
advertisement

材料科学基础
Fundamentals of Materials Science
Chapter 2
Fundamentals of Crystallology
Lan Yu
Faculty of Material Science and Engineering
Kunming University of Science and technology
References
1. E. J.Mittemeijer, Fundamentals of materials science. Springer-Verlag
Berlin Heideiberg 2010.
2. W.D. Callister, J.r, Foundations of materials science and engineering.
USA.5th-ed. John Wiley & Sons, Inc. 2001.
3. W.F. Smith, Foundations of materials science and engineering. New York,
McGraw-Hill book Co.1992.
4. C. Kittel, Introduction to solid state physics. USA. 8 th-ed. John Wiley &
Sons, Inc. 2005.
5. 周公度,结构与物性,第三版,高等教育出版社,2009.
6. 潘金生等,材料科学基础,修订版,北京,清华大学出版社,2011.
7. 胡赓祥,蔡珣等 材料科学基础,第三版,上海,上海交通大学出版社,
2010.
§2.3 Indices of crystal planes & directions
Ⅰ. What are crystal planes and directions ?
The atomic planes and directions passing through the crystal
are called (crystal) planes and directions respectively.
Ⅱ. Plane indices
1. Steps to determinate the plane indices:
① Establish a set of coordinate axes
② Find the intercepts of the planes to be indexed on a, b
and c axes (x, y, z).
c
z
y
x
a
b
③ Take the reciprocals of the intercepts 1/x, 1/y, 1/z.
④ Clear fractions but do not reduce to lowest integers.
⑤ Enclose them in parentheses, (h k l)
Example: 1/2,1,2/3
2,1,3/2
(423)
Plane indices referred to three axes a, b and c are also
called Miller Indices.
Several important points for the Miller indices of planes :
Planes and their negatives are identical. Therefore (020) (.020)
Planes and their multiples are not identical.
In cubic systems, a direction that has the same indices as a plane
is perpendicular to that plane.
2. The important planes in cubic crystals
(001)
(110)
(111)
(112)
3. A family of planes consists of equivalent planes
so far as the atom arrangement is concerned.
{110} (110) ( 1 10) (101)
(10 1 ) (011) (0 1 1)
Total: 6
{111} (111) ( 1 11) (1 1 1)
(11 1 )
Total: 4
{112} (112) ( 1 12) (1 1 2) (112)
(121) ( 1 21) (121) (12 1 )
(211) ( 211) (2 1 1) (21 1 )
Total: 12
{123} (123) ( 1 23) (123) (12 3) (132)
( 1 32) (1 3 2) (132) (231) ( 231)
(2 3 1) (23 1 ) (213) ( 213) (2 1 3)
(21 3) (312) ( 3 12) (3 1 2) (312)
(321) ( 3 21) (321) (32 1 )
Total: 4×3!=24
晶面族 {hkl}
晶体中具有相同条件(原子排列和晶面间距完全相同),空间位向不同的
各组晶面。用{hkl}表示。 如在立方晶胞中
(111),(111),(111),(111)同属
{111}晶面族。
Ⅲ. Direction Indices
1. Derivation for the crystallographic direction
①
Firstly, set a point on the indexed direction as the origin
point of coordinate axes.
②
Find the coordinates of another point on the indexed
direction: x,y,z.
③
Reduce x,y,z to three smallest integers: u, v, w.
④
Enclose in square brackets [u v w].
*指数看特征,正负看走向
(x1,y1,z1),(x2,y2,z2)二点连线的晶向指数:[x2-x1,y2-y1,z2-z1]
晶向族 <uvw>
晶体中原子排列情况相同但空间位向不同的一组晶向,用<uvw>表示。
在立方晶系里,数字相同,但排列顺序不同或正负号不同的晶向属于
同一晶向族。
如<100>=[100]+[001]+[010]
晶向族<u v w>
具有等同性能的晶向归并而成
2. The important direction in cubic crystals:
<100> : crystal axes
<110> : face diagonal
<111> : body diagonal
<112> : apices to opposite face-centers
[’eipisi:z] apex[’eipeks]
3. Family of directions consists of
crystallographically equivalent directions,
denoted <u v w>
e.g. 100 [100] [010] [001]
[ 1 00] [0 1 0] [00 1 ]
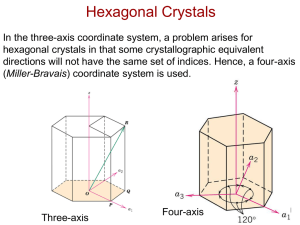
§2.4 Hexagonal axes for hexagonal
crystals
Ⅰ. Why choose four-axis system?
Four indices has been devised for hexagonal
unit cells because of the unique symmetry of the
system.
c
(1 1 0)
(100)
b
a
[100]
[110]
Ⅱ. Plane indices (hkil)
It can be proved: i ≡-(h+k)
(100) (10 1 0)
(1 1 0) (1 1 00)
Important planes :
c
(10 1 2)
(0001)
a3
(1120)
a2
a1
(10 1 1)
(10 1 0)
Ⅲ. Direction indices [ u v t w ]
To make the indices unique, an additional condition is
imposed. ---- Let t=-(u+v)
[0001]
[ 1 011]
Important directions
[01 1 0]
[2 1 1 0]
晶面指数:在四个轴上的截距,求倒数,整数化 (h k i l)
h+k+i=0
晶向指数:行走法,[u v t w],u+v+t=0
Transformation of indices
Transformation of 3 to 4 indices, or vice versa. Suppose
we have a vector, whose 3 indices [u v w], and 4 indices [u
v t w].
We have
Since
L ua1 va2 ta3 wc
Ua1 Va2 Wc
a3 (a1 a2 )
t (u v)
ua1 va 2 (u v)(a1 a2 ) wc
Ua1 Va2 Wc
(2u v)a1 (u 2v)a 2 wc
Ua1 Va2 Wc
U 2u v
V 2v u
u 1 3 (2U V )
v 1 3 (2V U )
or:
w W
t (u v)
W w
Examples and Discussions
1. Quick way for indexing the directions in cubic crystals:
The value of a direction depends on its feature while the
sign on direction.
2. The coordinate origin can be set arbitrarily (for
example on apices, body-center, face-centers etc.),
but never on plane in questions, otherwise the
intercepts would be 0,0,0 .
3. The coordinate system can be transferred arbitrarily,
but rotation is forbidden.
4. The atomic arrangement and planar density of the
important direction in cubic crystal.
plane
indices
BCC
atomic
atomic
planar density
arrangement
arrangement
planar density
a
1
4 1
a2
a2
1
4 1
2
4
a2
a2
2a
1
4 1
1.4
4
a2
2a 2
{100}
a
a
{110}
{111}
FCC
2a
2a
2a
4
1
6 0.58
a2
3 2
a
2
3
a
a
a
2a
2a
2a
2a
1
1
4 2
4
2 1.4
a2
2a 2
1
1
3 3
6
2 2.3
a2
3 2
a
2
5. The atomic arrangement and linear density of the
important direction in cubic crystal.
linear
indices
<100>
<110>
<111>
BCC
atomic
arrangement
FCC
linear density
1
21
a
a
2
a
1
2 0.7
a
2a
2
2a
3a
1
2 1
1.16
2
a
3a
atomic
arrangement
linear density
1
21
a
a
2
a
2a
3a
1
2 1
1.4
2
a
2a
1
2 0.58
a
3a
2
Exercise
1. Calculate the planar density and planar packing fraction for the
(010) and (020) planes cubic polonium, which has a lattice
parameter of 0.334nm.
Solution
planar density (010)
atom per face
area of face
1
0.334 2
8.96 1014 atoms/cm 2
packing fraction (010)
area of atoms per face
area of face
1 (r 2 )
0.79
2
( 2r )
However, no atoms are centered on the (020) planes. There fore,
the planar density and the planar packing fraction are both zero.
Thanks !