PowerPoint Slides
advertisement

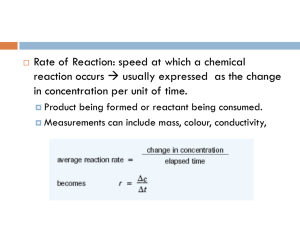
DCI 2.1 - Stoichiometry An atom of R weighs 1 mass unit (mu), G weighs 3 mass units (mu) and B weighs 2 mass units (mu). i) the mass of BG would be: ____mu ii) the mass of RG would be: ____mu Table II: Experiment #1. Ex p. #1 Formula REACTANTS Reactant(1) R REACTANTS Reactant(2) BG ---> PRODUCTS Product(1) RG PRODUCTS Product(2) B Initial Amount 4 4 0 0 Change Amount -4 -4 +4 +4 Final Amount 0 0 4 4 Table III Exp #1 1mu 5mu REACTANTS Reactant(2) BG ---> 4mu 2mu Ex p. #1 Formula REACTANTS Reactant(1) R PRODUCTS Product(1) RG PRODUCTS Product(2) B Initial mass 4 mu 20 mu 0 mu 0 mu Change in mass -4 mu -20 mu +16 mu +8 mu Final mass 0 0 16 mu 8 mu Table II: Exp #2 REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass 4 Change in mass Final mass Ex p. #2 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B 2 0 0 -2 -2 +2 +2 2 0 2 2 4mu 2mu ---> Complete Table III for Exp #2: Table III: Exp #2. 1mu 5mu REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass mu Change in mass Final mass Ex p. #2 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B mu mu mu mu mu mu mu mu mu mu mu ---> Table II: Exp #2 REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass 4 Change in mass Final mass Ex p. #2 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B 2 0 0 -2 -2 +2 +2 2 0 2 2 4mu 2mu ---> Complete Table III for Exp #2: Table III: Exp #2. 1mu 5mu REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass 4 mu Change in mass Final mass Ex p. #2 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B 10 mu 0 mu 0 mu -2 mu -10 mu +8 mu +4 mu 2 mu 0 mu 8 mu 4 mu ---> Table II: Exp #3 REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass 2 Change in mass Final mass Ex p. #3 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B 4 0 0 -2 -2 +2 +2 0 2 2 2 4mu 2mu ---> Complete Table III for Exp #3: Table III: Exp #3. 1mu 5mu REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass mu Change in mass Final mass Ex p. #3 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B mu mu mu mu mu mu mu mu mu mu mu ---> Table II: Exp #3 REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass 2 Change in mass Final mass Ex p. #3 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B 4 0 0 -2 -2 +2 +2 0 2 2 2 4mu 2mu ---> Complete Table III for Exp #3: Table III: Exp #3. 1mu 5mu REACTANTS Reactant(1) REACTANTS Reactant(2) R BG Initial mass 2 mu Change in mass Final mass Ex p. #3 Formula PRODUCTS Product(1) PRODUCTS Product(2) RG B 20 mu 0 mu 0 mu -2 mu -10 mu +8 mu +4 mu 0 mu 10 mu 8 mu 4 mu ---> Compare the results of Tables II and III. What is the relationship between the amounts of substances in each of the two tables? How is the set of numbers for the initial amounts related to the balanced chemical equation? Compare the results of Tables II and III. How is the set of numbers for the final amounts related to the balanced chemical equation? How is the set of numbers for the change amounts related to the balanced chemical equation? 1. Consider the following chemical equation describing the reaction between sulfur dioxide and oxygen. 2SO2 (g) + O2(g) → 2SO3(g) Given the following container as representing the final condition: Which of the containers (-->) best represents the initial conditions? 1. Consider the following chemical equation describing the reaction between sulfur dioxide and oxygen. 2SO2 (g) + O2(g) → 2SO3(g) Given the following container as representing the final condition: Which of the containers (-->) best represents the initial conditions? 2. Which of the following changes can be described by the balanced chemical equation, A2(g) + 3B2(g) → 2AB3(g) A) I only B) II only C) I and III D) II and III E) I, II and III 3. Which of the chemical equations best describes the reaction represented by the containers below? Consider the container label ‘initial condition’ as the reactants before any reaction has occurred, and the container labeled ‘final condition’ as the same container after the reaction has reached completion. A) B) C) D) E) 4A2(g) + 7B2(g) → 4AB3(g) 4A2(g) + 7B2(g) → 4AB3(g) + 1B2(g) + 2A2(g) A2(g) + 3B2(g) → 2AB3(g) 4A2(g) + 6B2(g) → 4AB3(g) A2(g) + B2(g) → AB3(g) 4. Consider the hypothetical reaction: 2G + R --> G2R If G has a mass = 3 mu and R = 2 mu What is in excess and what is the limiting reagent if 6 mu of R and 6 mu of G are mixed? A. R is the excess reagent and G is the limiting reagent: B. G is the excess reagent and R is the limiting reagent: C. both R and G are excess reagents: D. both R and G are limiting reagents