Limits and Excess Lesson PPT
advertisement

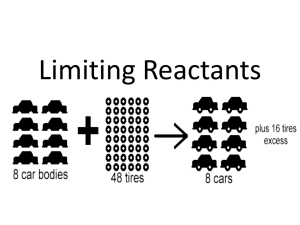
Stoichiometry Limiting and Excess Reactant Hayeon Jun Standards 3.3c A balanced chemical equation represents the conservation of atoms. The coefficients in a balanced equation can be used to determine mole ratios in the reaction. 3.3d The empirical formula of a compound is the simplest whole number ratio of atoms of the elements in a compound. It may be different from the molecular formula, which is the actual ratio of atoms in a molecule of that compound. 3.3f The percent composition by mass of each element in a compound can be calculated mathematically. Objectives At the completion of the following lessons, the students will be able to: - Balance equations given in the formulas for reactants and products - Calculate simple mole-mole stoichiometry problems, given a balanced equation - Determine the number of moles of a given substance, given its mass - Determine the mass of a given number of moles of a substance Agenda 1. Aim/Do-Now (5 minutes) 2. Do-Next (2 minutes) 3. Lab/Post-Lab (40 minutes) 4. Exit/Wrap-Up (2-3 minutes) Lab Report Aim: What are the limiting and excess reactants of our lab experiment? Do-Now Compare your pre-lab homework answers with your lab group. Discuss among group members if there are discrepancies. Previous Day Using the chart below, select your group number and the mass of aluminum metal and copper (II) chloride dihydrate you will be using in this activity: Group 1 2 3 4 5 6 Mass of Al (g) 1.00 0.50 0.50 0.50 0.25 0.25 Mass of CuCl2·2H2O (g) 2.00 2.00 4.00 6.00 5.00 4.00 Pre-lab (HW from the night before) 1. 2. 3. Aluminum metal reacts with aqueous copper (II) chloride dihydrate to form copper metal, aqueous aluminum chloride, and water. Write the balanced equation for this reaction. What does a solution look like when it contains dissolved copper (II) ions? For the balanced reaction, determine the mass of aluminum that would remain after a 10.00-g Al sample and 1.00 g of CuCl2·2H2O fully reacted. Show your work fully. Aim: What are the limiting and excess reactants of our lab experiment? Do-Next Get into your groups, gather all the materials needed for the lab, and begin your experiments! Materials: 250 mL beaker 100 mL graduated cylinder, spoon or stirring rod, watch glass, copper (II) chloride dihydrate, aluminum foil, water Lab Safety Precautions Wear safety goggles during this experiment. Avoid skin contact with copper (II) chloride dihydrate. Wash your hands before leaving the laboratory. Lab Procedures 1. 2. 3. 4. Weigh out the copper (II) chloride dihydrate in a 250 mL beaker. Using a graduated cylinder, measure out exactly 100 mL of water (tap water is fine) and add it to the beaker containing the copper (II) chloride dihydrate. Stir until the salt dissolves completely. Note the color of the solution in your lab notebook. Weigh out the appropriate mass of aluminum foil, crumple up into a few small pieces and add it to the copper (II) chloride dihydrate solution. Stir the solution gently and then allow it to sit undisturbed for 5 – 10 minutes. Write observations in your lab notebook. At the end of the period, label your beaker and cover it with a watch glass. Your teacher will designate an area to store your beakers overnight. During the next class period, you will be able to confirm the identity of the limiting reactant. Post-Lab 1. Indicate your group number. 2. What is the limiting reactant in this reaction? 3. What is the theoretical yield (in grams) of copper metal? 4. What is the theoretical yield (in grams) of aluminum chloride? 5. What mass of the excess reactant will remain when the limiting reactant is consumed? Limiting and Excess Reactants The limiting reactant is the reactant that is entirely consumed when a reaction goes to completion. The excess reactant is the reactant that is not completely consumed, and is left over. The moles of the product are always determined by the starting moles of the limiting reactant. Lab Report Include a brief introduction, data, the balanced reaction equation, and calculated results (including work). Determine the relative size of any error between your predicted post-reaction mass of the Al and its actual mass. Discuss possible causes for this error; be sure that the causes you discuss would result in the error you experienced, i.e. the actual post-reaction aluminum mass being larger or smaller than predicted.