FRET(Fluorescent Resonance Energy Transfer)
advertisement
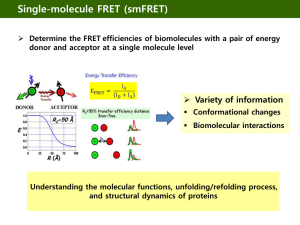
FRET(Fluorescent Resonance Energy Transfer) The problem • The use of fluorescent probes (ex. GFP) permits direct observation of the dynamic properties of specific proteins in live cells . • But, It is difficult to observe functional information like protein-protein interaction using fluorescent probes. • when proteins are labeled with different fluorophores, the optical resolution of light microscopes limits the detection of protein proximities to about 0.2 µm. What is FRET? • FRET is a process that shifts energy from an electronically excited molecule(donor) to a neighboring molecule(acceptor). • If the two fluorophores are close enogh, then excitation of the first molecule (Donor) results in fluorescence emission of the second molecule (Acceptor). What is FRET? CFP is in close to YFP. (1-10 nm) No FRET Signal •CFP is excited by light and emits light •CFP is more than 10 nm distant from YFP •YFP is not excited and does not emit light FRET Signal •CFP is excited by light but does emit little light •CFP is in close proximity (1-10 nm) to YFP •YFP is not excited by light but does emit light • transfer its excitation energy to a nearby acceptor chromophore in a non-radiative fashion through longrange dipole-dipole interactions. Fluorophore pair for FRET • The donor emission spectrum must overlap significantly with the acceptor excitation spectrum. Fluorophore pair for FRET • The excitation light for the donor must not directly excite the acceptor. Donor Excitation Acceptor Excitation CFP 440nm 480nm YFP 520nm 535nm BFP 365nm 460nm GFP 488nm 535nm CFP 440nm 480nm dsRed1 560nm 610nm FITC 488nm 535nm Cy3 525nm 595nm Cy3 525nm 595nm Cy5 633nm 695nm GFP 488nm 535nm Rhodamine 543nm 595nm Donor Emission Donor Acceptor Emission Acceptor • A donor can directly transfer its excitation energy to an acceptor through long-range dipole-dipole intermolecular coupling. – A theory proposed by Theodor Förster in the late 1940s – Resonance energy transfer is a non-radiative quantum mechanical process (no collision, heat) When energy transfer occurs, the acceptor molecule quenches the donor molecule fluorescence, and if the acceptor is itself a fluorochrome, increased or sensitized fluorescence emission is observed Rate constant , K • Rate constant for energy transfer(Kt) • 𝑄𝐷𝐽 𝐾𝑡 ~ 𝜏𝐷 × 1 𝑅6 × 𝜅2 – 𝐾𝑡 transfer rate – 𝑄𝐷 quantum yield of donor – 𝐽 spectral overlap of donor emission and acceptor absorption – 𝜏𝐷 fluorescence lifetime of donor – R distance between donor and acceptor – 𝜅2 orientational factor FRET Efficiency • Efficiency of Energy Transfer 𝑑𝑒𝑎𝑐𝑡𝑖𝑣𝑎𝑡𝑖𝑜𝑛 𝑟𝑎𝑡𝑒 𝑏𝑦 𝑒𝑛𝑒𝑟𝑔𝑦 𝑡𝑟𝑎𝑛𝑠𝑓𝑒𝑟 = 𝑑𝑒𝑎𝑐𝑡𝑖𝑣𝑎𝑡𝑖𝑜𝑛 𝑟𝑎𝑡𝑒 𝑏𝑦 𝑎𝑙𝑙 𝑜𝑓 𝑡ℎ𝑒 𝑝𝑎𝑡ℎ𝑤𝑎𝑦𝑠 E = kT / (kT + kr + knr) kT = rate of transfer of excitation energy kr = rate of fluorescence(radiation) knr = sum of the rates of all other deexcitation processes (nonradiation) R0 determination • E = kT / (kT + kr + knr) • Let, • R0 : distance between the donor and the acceptor at which 50% of FRET efficiency takes place. Then, E = 1 1+ 𝑅 6 𝑅0 R0 (Forster distance) = 9.78 x 103(n-4*fd*k2*J)1/6 Å depends on J(spectral overlap) Structural design of various FRETbased biosensors. a. The interaction of two proteins can be dynamically detected by FRET b. Molecular cleavage by protease will be translated into loss of FRET. c. An intramolecular probe consists of sandwiching two domains between CFP and YFP, which can interact after phosphorylation or binding to calcium, resulting in a change in FRET. d. An intramolecular probe consists of CFP, YFP and a protein/domain, which permits conformational change by binding to another biomolecule, leading to a change in FRET FRET quantification • FRET quantification is mostly based on measuring changes in fluorescence intensity or fluorescence lifetime upon changing the experimental conditions. Ex>a microscope image of donor emission with normal acceptor and with acceptor bleached. Various FRETs • CFP-YFP pairs -most popular FRET pair for biological use. -CFP(cyan fluorescent protein) -YFP(yellow fluorescent protein) both are GFP variants. They can be easily attached to a host protein by genetic engineering Various FRETs • BRET(Bioluminescence Resonance Energy Transfer) -FRET require external illumination to initiate the fluorescence transfer. -BRET use bioluminescent luciferase rather than CFP(donor) to produce an initial emission compatible with YFP. Various FRETs • Homo FRET -to examine the interactions between two, or more proteins of the same type. -both the acceptor and donor protein emit light with the same wavelenths. -by FRET anisotropy imaging, measuring FRET is available.