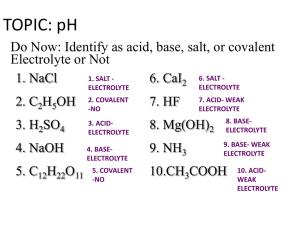
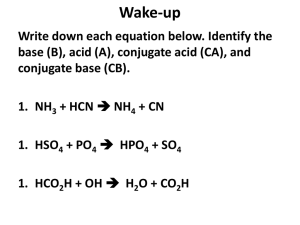
pH + pOH = 14
advertisement
Unit: Acids, Bases, and Solutions Calculations with Acids and Bases After today you will be able to… • Explain the correlation to strength of acids and bases to pH and pOH scale • Calculate pH, pOH, [H+], and [OH-] pH Scale pH scale: the measure of acidity of a solution acidic 0 neutral basic 7 + pH=-log[H ] [H+] = concentration in Molarity 14 Before we try an example, you will need to locate the “log” button on your calculator. Example: What is the pH of a solution that has an [H+]=1.5x10-4M? -4 pH=-log[1.5x10 ] pH=3.8 Example: To do this calculation + What is the [Hwill ] in need a solution you to use with pH=9.42? the inverse log. + 9.42=-log[H ] the “10x” Locate +] Usually it is button. -9.42=log[H 10-9.42the =[H+]second function of the -10 log [H+]=3.80x10 M button. pH Scale pOH scale: the measure of alkalinity (basic-ness) of a solution basic 0 neutral acidic 7 pOH=-log[OH ] 14 Example: What is the pOH of a solution that has an [OH-]=3.27x10-9M? -9 pOH=-log[3.27x10 ] pOH=8.49 Since the pH and pOH scales are opposite each other: pH + pOH = 14 Example: What is the pH in a solution with a pOH=8.6? pH + 8.6 = 14 pH=5.4 Summary… + pH=-log[H ] pOH=-log[OH ] pH + pOH = 14 Ion-Product Constant for Water Water will self-ionize to a certain extent into its individual ions. Because of this, the following relationship can be used: + -14 [H ][OH ]=1.0x10 M Kw Example: + [H ] What is the in a solution with [OH-] = 6.73x10-5M? + -5 -14 [H ][6.73x10 ]=1.0x10 [H+] = 1.49x10-10M Sometimes multiple formulas must be used to carry out these calculations: Example: What is the pOH in a solution with an [H+] = 2.17x10-5M? (Note: there are multiple ways to do this problem!) [H+][OH-]=1.0x10-14 [2.17x10-5][OH-]=1.0x10-14 [OH-]=4.61x10-10M + pH=-log[H ] pOH=-log[OH ] pH + pOH = 14 + -14 [H ][OHpOH=9.34 ]=1.0x10 pOH=-log[OH-] pOH=-log[4.61x10-10] Example: What is the [OH-] in a solution with pH=8.1? (Note: there are multiple ways to do this problem!) pH + pOH = 14 8.1 + pOH = 14 pOH = 5.9 + pH=-log[H ] pOH=-log[OH ] pH + pOH = 14 + -14 [H ][OH ]=1.0x10 [OH ]=1.3x10 M pOH=-log[OH-] 5.9=-log[OH-] - -6 Questions? Begin WS4