Density
advertisement

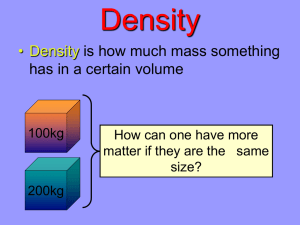
Q) Which weighs more:A kilogram of feathers or a kilogram of iron? DENSITY What is Density? If you take the same volume of different substances, then they will weigh different amounts. Wood Water Iron 1 cm3 1 cm3 1 cm3 0.50 g 1.00 g 8.00 g IRON Q) Which has the greatest mass and therefore the most dense? Density is the Mass per unit Volume Cube Representations 1 m3 = 1 000 000 cm3 Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 119 Density Equation: g Density = Mass Volume g/cm3 cm3 d= m V d = m = 18.9g = 16.88 g/cm3 V 1.12cm3 No – it is not pure gold! Example: Q) A gold-colored ring has a mass of 18.9 grams and a volume of 1.12 cm3. Is the ring pure gold? (The density of gold is 19.3 g/ cm3.) DENSITY OF A REGULAR SOLID Find the Mass of the solid on a balance. 2.0 cm Measure the three lengths and calculate the Volume. d = m = 240 =10.0 g/cm3 (i.e., V = l x w x h ) V 24 3.0 cm (2cm * 3cm * 4cm = 24 cm3) Calculate the Density. m = 240 g 4.0 cm DENSITY OF AN IRREGULAR SOLID m = 360 g 80 cm3 50 cm3 d = m = 360 =12.0 g/cm3 V 30 Find the Mass of the solid on a balance. Fill the Measuring Cylinder with Water to a known Volume. Add the Object. Work out the Volume of Water that is displaced. Calculate the Density. What happens when you put an object in a fluid? If the object is less dense than the fluid… it floats What happens when you put an object in a fluid? If the object is more dense than the fluid… it sinks! What happens when you put an object in a fluid? If the object is as dense as the fluid… Neither floats nor sinks!