Units of Measurements - Belle Vernon Area School District
advertisement

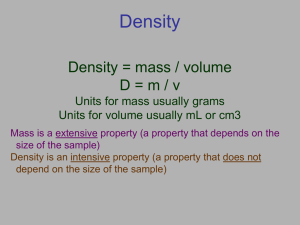
Units of Measurement 2.2 Definitions Weight –measure of the gravitational pull on matter Diff between mass and weight Quantity – something that has magnitude, size or amount Every measurement must have a unit SI-Le Système International d’Unitès We use 7 base units and prefixes to express all units Quantity Length Symbol Unit l Meter Abbreviation m Mass m Kilogram kg Time t Second s Thermodynamic Temperature Amount of a Substance Electric Current Luminous Velocity T Kelvin k n Mole mol I Iυ Ampere Candela A cd Mega - M 1 000 000 (b.u.) = 1M(b.u.) Kilo - k 1 000 (b.u.) = 1k(b.u.) hecto - h 100 (b.u.) = 1h(b.u.) deka - da 10 (b.u.) = 1da(b.u.) Base Unit deci - d 10 d(b.u.) = 1 (b.u.) centi - c 100 c(b.u.) = 1 (b.u.) milli - m 1 000 m(b.u.) = 1 (b.u.) micro - µ 1 000 000 µ(b.u.) = 1 (b.u.) nano - n1 000 000 000 n(b.u.)=1 (b.u.) Derived Units Combination of SI base units Examples – m3, g/cm3, kg/mol Volume – the amount of space occupied by an object 1000mL = 1 L = 1000cm3 Conversion Factors Ratio derived from the equality b/t 2 different units that can be used to convert from 1 unit to another Ex: there are 4 quarters in 1 dollar Dimensional analysis – math technique that allows you to use units to solve problems involving measurments Conversion Factors Quarters and dollars conversion factor – 4 quarters = 1 dollar Convert 18 quarters to dollars Start with what you know What you are going to goes in the top and coming from goes in the bottom Convert 3 dollars to quarters Conversions Convert 45 minutes to hours. Convert 2.5 hours to minutes. Convert 100 millimeters to meters 1 meter = 1000millimeters Convert 3 hours to seconds Conversions Convert 0.12 kg to mg Must convert to base unit first if going from one side to the other. Convert 130 cm to Mm Conversions with Derived Units Convert 60 miles/hour to kilometers per second 1 mile = 1.609 kilometers How many grams per milliliter are there in 34 kilograms per liter Density The ratio of mass to volume D = m Know this v equation!!! Typically expressed as g/cm3. Predict the order of the following from the most dense to the lease dense… Milk, mercury, gasoline, ice, a diamond, a cork Density Silver Bracelet Mass is 100.0 g and when it displaces water, volume is 20.5 cm3 Density of silver is 10.5 g/cm3 Is this a silver bracelet? Density Calculations A piece of an unknown material has a mass of 5.85 g and a volume of 7.57 cm3. What is the density of the material? Density Calculations D = m v D = m = 5.85 g v 7.57 cm3 Density = 0.77 g/cm3 Density calculations A 200 g piece of metal has a volume of 25 cm3. Calculate the density of the metal. An unknown mass of silicon occupies a volume of 350 cm3. Calculate the mass of Si knowing that the density of Si is 2.34 g/cm3. Density What is the volume that a 50 gram piece of gold occupies? The density of gold is 19.30g/cm3. Density A block of lead with dimensions of 2.0dm x 8.0cm x 35mm, has a mass of 6.356kg. Calculate the density of lead in g/cm3. Density A flask weighs 345.8 g and is filled with 225 mL of carbon tetrachloride. The weight of the flask and carbon tetrachloride is found to be 703.55 g. Calculate the density in g/ml and g/L Density A 28.5 g of iron shot is added to a graduated cylinder containing 45.5 mL of water. The water level rises to the 49.1 mL mark. Calculate the density. Density A cylindrical tube of length 27.75 cm and radius of 2.00 cm is filled with argon gas. The empty tube weighs 188.25g. The tube filled with argon weighs 188.87g . Calculate the density. Assignment Complete the section review on page 42 and do numbers 1-5. This is due tomorrow!