Quantum Mechanics

Alright class we are going back to quantum numbers.
I decided this would be a better lead into dipole then just throwing you into the mess.
Do you remember Wave and
Frequency?
• Do you remember plank. Well he is back it is time to work on his constant and what it means.
• Also the electromagnetic spectrum.
Properties of Waves
Wavelength ( l
) is the distance between identical points on successive waves.
Amplitude is the vertical distance from the midline of a wave to the peak or trough.
Frequency ( n
) is the number of waves that pass through a particular point in 1 second (Hz = 1 cycle/s).
The speed ( u ) of the wave = l x n
3
Maxwell (1873), proposed that visible light consists of electromagnetic waves .
Electromagnetic radiation is the emission and transmission of energy in the form of electromagnetic waves.
Speed of light ( c ) in vacuum = 3.00 x 10 8 m/s
All electromagnetic radiation l x n = c
4
5
A photon has a frequency of 6.0 x 10 4 Hz. Convert this frequency into wavelength (nm). Does this frequency fall in the visible region?
6
The wavelength of the green light from a traffic signal is centered at 522 nm. What is the frequency of this radiation?
7
Problem 1
What is the wavelength (in meters) of an electromagnetic wave whose frequency is
3.64 x 10 7 Hz?
8
Mystery #1, “Heated Solids Problem”
Solved by Planck in 1900
When solids are heated, they emit electromagnetic radiation over a wide range of wavelengths.
Radiant energy emitted by an object at a certain temperature depends on its wavelength.
Energy (light) is emitted or absorbed in discrete units
(quantum).
E = h x n
Planck’s constant (h) h = 6.63 x 10 -34 J • s
9
Calculate the energy (in joules) of a photons with a wavelength of 5.00 x 10 4 nm (infrared region).
10
Calculate the energy (in joules) of a photons with a wavelength of 5.00 x 10 -2 nm (x-ray region).
11
Problem 2
The energy of a photon is 5.87 x 10 -20 J. What is its wavelength in nanometers?
12
Mystery #2, “Photoelectric Effect”
Solved by Einstein in 1905
Light has both:
1. wave nature
2. particle nature
Photon is a “particle” of light h n
= KE + W
KE = h n
W where W is the work function and depends how strongly electrons are held in the metal h n
KE e -
13
When copper is bombarded with high-energy electrons, X rays are emitted. Calculate the energy (in joules) associated with the photons if the wavelength of the X rays is 0.154 nm.
14
The work function of cesium metal is 3.42 x 10 -19 J. Calculate the minimum frequency of light necessary to eject electrons from the metal.
15
The work function of cesium metal is 3.42 x 10 -19 J. Calculate the kinetic energy of the ejected electron if light of frequency
1.00 x 10 15 s -1 is used for irradiating the metal.
16
Problem 3
The work function of titanium metal is 6.93 x
10 -19 J. Calculate the energy of the ejected electrons if light of frequency 2.50 x 10 15 s -1 is used to irradiate the metal.
KE = _____x 10 -19 J
17
Mystery #3, “Emission Spectra”
Line Emission Spectrum of Hydrogen Atoms
18
19
Bohr’s Model of the Atom (1913)
1. e can only have specific
(quantized) energy values
2. light is emitted as e moves from one energy level to a lower energy level
E n
= R
H
1
( ) n 2 n (principal quantum number) = 1,2,3,…
R
H
(Rydberg constant) = 2.18 x 10 -18 J
20
E = h n
E = h n
21
n i
= 3 n i
= 3 n i
= 2 n f
= 2 n n f f
E photon
=
D
E = E f
E i
E f
= R
H
1
( ) n f
2
E i
= R
H
1
( ) n i
2
D
E = R
H
1 n 2 i n
1
( ) f
22
23
Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 state to the n = 3 state.
24
Problem 4
What is the wavelength (in nm) of a photon emitted during a transition from n i in the H atom?
=6 to n f
=4
ν = _____x 10 3 nm
25
Why is e energy quantized?
De Broglie (1924) reasoned that e is both particle and wave .
2 p r = n l l
= h mu u = velocity of em = mass of e-
26
What is the de Broglie wavelength (in nm) associated with a 2.5 g Ping-Pong ball traveling at 15.6 m/s?
27
Calculate the wavelength of the ‘particle’ in each of the following two cases: (a) The fastest serve ever recorded in tennis was about 150 miles per hour, or
68 m/s. Calculate the wavelength associated with a
6.0 x 10 -2 kg tennis ball travelling at this speed.
28
Calculate the wavelength of the ‘particle’ in each of the following two cases: (b) Calculate the wavelength associated with an electron (9.1094 x 10 -31 kg) travelling at this speed.
29
Problem 5
Calculate the wavelength (in nm) of a H atom (mass = 1.674 x 10 -27 kg) moving at
7.00 x 10 2 cm/s.
30
Chemistry in Action: Laser – The Splendid Light
L ight A mplification by S timulated E mission of R adiation
Laser light is (1) intense, (2) monoenergetic, and (3) coherent
31
Chemistry in Action: Electron Microscopy l e
= 0.004 nm
STM image of iron atoms on copper surface
Electron micrograph of a normal red blood cell and a sickled red blood cell from the same person
32
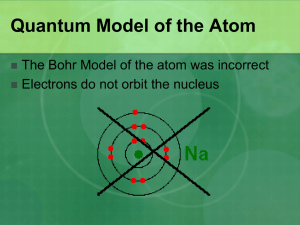
Shortcomings of Bohr’s model
•Did not account for the emission spectra of atoms containing more than on electron.
•Did not explain extra lines in the emission spectra for hydrogen when magnetic field is applied.
•Conflict with discovery of ‘wavelike’ properties – how can you define the location of a wave?
Heisenberg Uncertainty Principle
It is impossible to know simultaneously both the momentum p (defined as mass times velocity) and the position of a particle with certainty.
33
Schrodinger Wave Equation
In 1926 Schrodinger wrote an equation that described both the particle and wave nature of the e -
8 p h
2
2 m
2
V
( r , t )
= iH
2 p
( r , t )
t
34
Schrodinger Wave Equation
Wave function (
) describes:
1. energy of e with a given
2. probability of finding e in a volume of space
Schrodinger’s equation can only be solved exactly for the hydrogen atom. Must approximate its solution for multi-electron systems.
35
Schrodinger Wave Equation
is a function of four numbers called quantum numbers ( n , l , m l
, m s
) principal quantum number n n = 1, 2, 3, 4, ….
distance of e from the nucleus n =1 n =2 n =3
36
Where 90% of the e density is found for the 1s orbital
37
Schrodinger Wave Equation quantum numbers : ( n , l , m l
, m s
) angular momentum quantum number l for a given value of n, l = 0, 1, 2, 3, … n -1 n = 1, l = 0 n = 2, l = 0 or 1 n = 3, l = 0, 1, or 2 l = 0 s orbital l = 1 p orbital l = 2 d orbital l = 3 f orbital
Shape of the “volume” of space that the e occupies
38
l = 0 ( s orbitals) l = 1 ( p orbitals)
39
l = 2 ( d orbitals)
40
Schrodinger Wave Equation quantum numbers : ( n , l , m l
, m s
) magnetic quantum number m l for a given value of l m l
= l , …., 0, …. + l if l if l = 1 (p orbital), m l
= 2 (d orbital), m l
= -1, 0, or 1
= -2, -1, 0, 1, or 2 orientation of the orbital in space
41
m l
= -1, 0, or 1 3 orientations is space
42
m l
= -2, -1, 0, 1, or 2 5 orientations is space
43
Schrodinger Wave Equation
( n , l , m l
, m s
) spin quantum number m s m s
= + ½ or ½ m s
= + ½ m s
= ½
44
Schrodinger Wave Equation quantum numbers : ( n , l , m l
, m s
)
Existence (and energy) of electron in atom is described by its unique wave function
.
Pauli exclusion principle - no two electrons in an atom can have the same four quantum numbers.
Each seat is uniquely identified (E, R12, S8)
Each seat can hold only one individual at a time
45
46
Schrodinger Wave Equation quantum numbers : ( n , l , m l
, m s
)
Shell – electrons with the same value of n
Subshell – electrons with the same values of n and l
Orbital – electrons with the same values of n, l , and m l
How many electrons can an orbital hold?
47
How many 2 p orbitals are there in an atom?
How many electrons can be placed in the 3 d subshell?
48
List the values of n, ℓ, and m ℓ for orbitals in the 4d subshell.
What is the total number of orbitals associated with the principle quantum number n=3?
49
Problem 6
Give the values of the quantum numbers associated with the orbitals in the 3p subshell.
n = ____ ℓ = ____ m ℓ
=____
50
Problem 7
What is the total number of orbitals associated with the principle quantum number n=4.
51
Energy of orbitals in a single electron atom
Energy only depends on principal quantum number n
52
Energy of orbitals in a multi -electron atom
Energy depends on n and l
53
“Fill up” electrons in lowest energy orbitals ( Aufbau principle )
54
The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins ( Hund’s rule ) .
55
Order of orbitals (filling) in multi-electron atom
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s
56
Write the four quantum numbers for an electron in a 3p orbital.
57
Problem 8
Write the four quantum numbers for an electron in a 4d orbital.
58
Electron configuration is how the electrons are distributed among the various atomic orbitals in an atom.
number of electrons in the orbital or subshell
1s 1 principal quantum number n angular momentum quantum number l
Orbital diagram
H
1s 1
59
What is the electron configuration of Mg?
What are the possible quantum numbers for the last
(outermost) electron in Cl?
60
What is the maximum number of electrons that can be present in the principle level for which n=3?
61
Problem 9
Calculate the total number of electrons that can be present in the principle level for which n=4
62
An oxygen atom has a total of eight electrons. Write the four quantum numbers for each of the electrons in the ground state..
63
Problem 10
Write a complete set of quantum numbers for each of the electrons in boron.
64
Paramagnetic unpaired electrons
2p
Diamagnetic all electrons paired
2p
65
Outermost subshell being filled with electrons
66
67
Write the ground state electron configuration for sulfur
Write the ground state electron configuration for palladium which is diamagnetic
68
Problem 11
Write the ground state configuration for phosphorus.
69