Mr. Cherry`s Chapter 5 Notes
advertisement

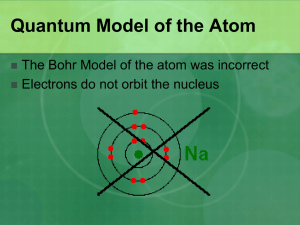
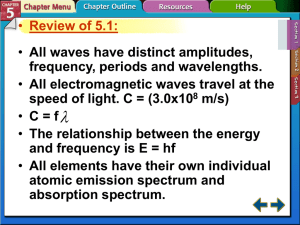
Electrons in Atoms Chapter 5 Duality of Light Einstein proved that matter and energy are related E = mc2 The smaller matter becomes the more like energy it behaves. We know electrons are very small therefore behave like energy. Duality of Light During the 1800’s, Chemists and physicists found light emitted properties of a wave. In 1905, Albert Einstein explained the photoelectric effect using a particle theory of light. Scientist have accepted a dual theory of light, either wave or particle. Wave Nature of Light Electromagnetic Radiation: Form of energy that exhibits wavelike behavior as it travels through space. Visible light is a form of electromagnetic radiation. All electromagnetic radiation travels at 3.00 x 108 m/s Wave Nature of Light Crest Wave Nature of Light Wavelength: represented by λ, is the shortest distance between equivalent points. Unit for wavelength is meter. Frequency: represented by ν Greek letter nu, is the number of waves that pass a given point per second. Unit for frequency is s-1 or 1/s Wave Nature of Light Wave Nature of Light Since all electromagnetic radiation travels at 3.00 x 108 m/s, there is a distinct relationship between frequency and wavelength. c=λν c is a constant, 3.00 x 108 m/s, the speed of light λ is the wavelength of light in meters ν is the frequency in s-1 or 1/s or Hz which = s-1 or 1/s Wave Nature of Light Microwaves are used to transmit information. What is the wavelength having a frequency of 3.44 x 109 Hz We know c = λ ν We know c = 3.00 x 108 m/s and we know ν = 3.44 x 109 Hz Solve for λ λ = c/ ν λ = 3.00 x 108 m/s/ 3.44 x 109 Hz λ = .0872m or 8.72 x 10-2 m Wave Nature of Light An X ray has a wavelength of 1.15 x 10-10 m. What is the frequency? 2.61 s-1 Yellow light has a frequency of 5.09 x 1014 s-1. What is the frequency? 5.89 x 10-7 m. Particle Nature of Light Max Plank did experiments studying a black box and the heat given off by it. Plank determined energy can only be released or absorbed by atoms in small specific amounts called quanta. He suggested light consisted of photons. Particle Nature of Light Plank proposed the energy of a single quantum is E=hν E is the energy of a quantum. h is Planck’s constant, 6.626 x 10-34 J x s, J is the symbol of a joule, which is the SI unit of energy. ν is frequency. Particle Nature of Light Calculate the energy of a photon with a frequency of 7.23 x 1014 s-1 . We know E = h ν We know h = 6.626 x 10-34 J x s We know v = 7.23 x 1014 s-1 E = 6.626 x 10-34 J x s x 7.23 x 1014 s-1 E = 4.79 x 10-19 J Particle Nature of Light Calculate the energy of a photon with the frequency of 6.32 x 1020 s-1. 4.19x 10-13 J Calculate the energy of a photon with a wavelength of 5.98 x 10-7 m. 3.32 x 10-19 J Relationships of Photons v↑, E↑, λ↓ v↓, E↓. λ↑ Atomic Emission Spectra Neon lights Atoms absorb energy and emit light to release energy Atomic emission spectrum: set of frequencies of the electromagnetic waves emitted by atoms of the element. Unique for all different elements. Electrons as Waves de Broglie proposed electrons have wave like nature and the wavelength of the electron can be calculated λ = h/mv h = Planck’s constant m = mass v = velocity Bohr Model of the Atom Bohr built upon Planck’s and Einstein’s concepts of quantized energy. The chemical properties of the element are determined by the number of electrons in the outer orbits. Used spectra from hydrogen atom Proposed that hydrogen atom has only certain allowable energy states Ground State: Lowest allowable energy state Stated electron moves in only certain circular orbits. Bohr Model of the Atom The smaller the electron’s orbit, the lower the atom’s energy state. Larger orbit, higher energy state. First orbit closest to the nucleus, n=1, second, n=2 Bohr Model of the Atom When energy is added to an atom, the electron moves from the ground state, n=1, to a higher energy orbit, excited state, n=2 When an electron is in an excited state, it can drop from the higher energy orbit to a lower energy orbit. The atom emits a photon corresponding to the difference between the corresponding energy levels. Bohr Model of the Atom 1. proposed that while circling the nucleus of the atom, electrons could only occupy certain discrete orbits, that is to say energy levels 2. electrons give or take energy only when they change their energy levels. If they move up, they take energy if they move down, they release energy. This energy itself is released in discrete packets called photons 3. an electron which is not in its native energy level always has to fall back to its original, stable level. Bohr Model of the Atom Bohr model perfectly explained the hydrogen atom but, failed to explain any of the other atoms. Therefore, the Bohr model of the atom is not the currently accepted model of the atom. Heisenberg Uncertainty Principle It is impossible to measure an object without disturbing the object. Heisenberg attempted to determine exact location of electrons using photons. He found since photons and electrons have relatively the same amount of kinetic energy, the photon would disturb the position and velocity of the electron. Heisenberg Uncertainty Principle It is impossible to know precisely both the velocity and position of a particle at the same time Quantum Mechanical Model of Atom Schrödinger created an equation to accurately determine where an electron is. Unlike Bohr model, makes no attempt to determine orbit of electron. States electrons are in noncircular orbitals. Orbitals are based on probability of location of electron. Atomic Orbitals Principal quantum numbers (n): indicate the relative sizes and energies of atomic orbitals. As n increases, the orbital is further away from the nucleus and has more energy. n has whole number values of 1, 2, 3, etc. Atomic Orbitals Principle energy levels contain energy sublevels. Sublevels are signified by letters s, p, d, or f. The letter determines the shape of the sublevel. S orbital First found at energy level 1 Holds 2 electrons Spherical 1s, 2s, 3s,… P Orbital First found at energy level 2 3 different shapes per energy level 2 electrons per shape “dumb-bell” shaped D Orbitals First found at energy level 3. 5 different shapes 2 electrons per shape Complex shapes F Orbitals First found at energy level 4 7 different shapes 2 electrons per shape Very complex f Orbital First Four Principal Energy Levels Principle Quantum Number Sublevels Present Number of orbitals in each sublevel Total number of electrons in energy level 1 s 1 2 2 s p 1 3 8 3 s p d 1 3 5 18 4 s p d f 1 3 5 7 32 Quantum Numbers Principle quantum number = n 2nd or Azinmuthal quantum number = describes shape 0, 1, 2, 3 (s, p, d, f) Magnetic quantum number = ml orientation in space, whole numbers -1, 0, 1 Spin = s, either ½ or – ½, one e- ½ other e- has – ½. n Azinmuth numbers 1 Number of orbitals Values of ml S values Number of orbitals in energy level 0 0 ½, - ½ 1 2 0 1 0 -1,0,1 ½, - ½ 4 3 0 1 2 0 -1,0,1 -2,-1,0,1,2 ½, - ½ 9 4 0 1 2 3 0 -1,0,1 -2,-1,0,1,2 -3,-2,-1,0,1,2,3 ½, - ½ 16