Ch 5 - Electrons in Atoms
advertisement

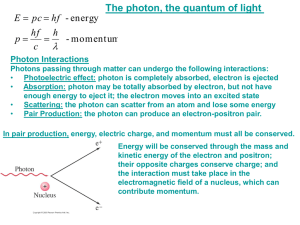
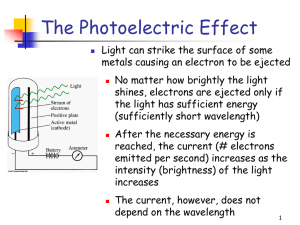
Ch 5 Electrons in Atoms Light and Quantized Energy Light • The study of light led to the development of the quantum mechanical model • Light is a kind of electromagnetic radiation • Electromagnetic radiation includes many kinds of waves • All move at 3.00x108 m/s or 3.00x1010 cm/s (abbreviated: c) c = ln Parts of a wave Parts of a Wave • • • • • Origin - the base line of the energy Crest - high point on a wave Trough - low point on a wave Amplitude - distance from origin to crest Wavelength – shortest distance between equivalent points on a continuous wave l • abbreviated = Greek letter lambda • Usually measured in meters, or in the case of electromagnetic radiation, nanometers (1 nm = 1x10-9m) Frequency • The number of waves that pass a given point per second. • Units are cycles/sec, 1/s, s-1 or hertz (Hz) • Abbreviated n - the Greek letter “nu” Frequency and Wavelength • Inversely related • As one goes up the other goes down. • Different frequencies of light are different colors of light. • There is a wide variety of frequencies • The whole range is called a spectrum Electromagnetic Spectrum High Low energy energy Radio Micro Infrared Ultra- XGamma waves waves violet Rays Rays Low High Frequency Frequency Long Short Wavelength Wavelength Visible Light Wave Equation Example Problems • A light has a wavelength of 550 nm. What is its frequency? 5.45x1014Hz • A light has a frequency of 4.25x1014 Hz. What is its wavelength in nanometers? What about meters? 706 nm 7.06x10-7m Particle Nature of Light • Understanding light as a wave does not explain all of light’s interactions with matter. • People knew that when you heat up some objects they change to specific colors • Ex: Heating up an electric stove top burner. • As the metal gets hotter it has more energy and gives off different colors • 1900, German physicist Max Planck started researching a way to explain this phenomenon Light is Quantized Energy • Energy is quantized. • It can be lost or gained only in specific amounts. • Light is energy. • Therefore light must be quantized. • Matter can only gain or lose energy in these specific amounts called quanta (plural for quantum) • A quantum is the certain amount of energy that can be gained or lost by an atom. • You cannot have amount that are fractions of a quantum. • Think about walking up stairs – you cannot walk up or down a fraction of a stair Energy and Frequency • Max Planck developed a way to describe this property of light mathematically. • Energy and frequency are directly related • E = hn • E is the energy of the quantum (Units of Joules) • n is the frequency (Units of Hz) • h is Planck’s constant; h = 6.626 x 10 -34 Js Einstein used this theory to explain why metals (and some semi-metals) will eject e- from the surface when light of specific a frequency hits it. • This is called the photoelectric effect • Used with solar panels • The metal will not eject the e- if the frequency is too low. • The energy has to reach a threshold frequency (minimum amount) for that particular substance Einstein’s Idea • 1905, Einstein went on to further say that light is made of particles • These smallest pieces (particles) of “electromagnetic radiation” (light) are called photons and carry a quantum of energy • Light can act as both waves and particles = wave-particle duality of light Example 1 • What is the energy of a photon of radiation with a frequency of 7.23 x 1014 Hz? E = 4.79 x 10 -19 J Example 2 • What is the energy of a photon of radiation with a frequency of 6.32 x 1020 Hz? E = 4.19 x 10 -13 J Example 3 • What is the frequency of a photon of radiation with an energy of 6.96 x 10 -18 J? If a metal needs an energy of 5.45 x 10-17 J to emit electrons will it happen with this photon? n = 1.05 x 1016 Hz Example 4 • What is the energy of a photon with a wavelength of 4.00 x 10-7 m? E = 4.97 x 10-19J Atomic Spectra How light & color tells us about atoms Prism • White light is made up of all the colors of the visible spectrum. • Passing it through a prism separates it. If the light is not white • By electrifying a gas (like Neon) or exciting other elements with enough energy scientists can get it to give off colors. • Passing this light through a prism or diffraction grating does something different. Atomic Spectrum • Each element gives off its own characteristic colors. • Can be used to identify the atom. • Like “atomic fingerprints” • Used to I.D. elements stars are made of • Used to I.D. unknown elements in compounds Atomic Spectrum • Color is given off only when electrons are LOSING energy so they can return to a more stable state • These are called Line Spectra • Made from emission of light (also called emission spectra) • Unique to each http://jersey.uoregon.edu/elements/Elements.html element. 5.2 Quantum Theory & The Atom An explanation of Atomic Spectra Bohr’s Model • Niels Bohr – Danish physicist – proposed model to explain the emission spectra of elements – 1922 received Nobel Prize in Physics • Electrons move like planets around the sun in circular orbits at different levels. • Energy separates one level from another. • The smaller the orbit, the lower the energy Bohr’s Model Nucleus Electron Orbit Energy Levels Bohr’s Model Increasing energy Fifth Fourth Third Second First Nucleus Further away from the nucleus the electron is means it has more energy. There is no “in between” energy Chemists call these the electrons’ Energy Levels Where the electron starts The energy level an electron starts from is called its ground state. • Let’s look at a hydrogen atom Electron’s ground state Changing the energy • Heat, electricity or light can move the electron up energy levels • How much it moves depends on amount of energy absorbed • As the electron falls back to ground state it gives the energy back as light. • Electrons may fall down in steps • Each step has a different energy Hydrogen atom’s atomic n=4 emission n=3 n=2 n=1 36 Getting these wavelengths you can calculate the Energy • We can calculate the energy the electrons had: DE = E high-energy orbit – E lower-energy orbit • Don’t forget that E=hn • Further the e- fall, the more energy, so higher frequency. • The electrons can emit or absorb only certain amounts of energy There is one issue with this model: • Unfortunately this model’s calculations ONLY worked for Hydrogen b/c Bohr didn’t understand how electrons actually moved in an atom The Quantum Mechanical Model of the Atom is born! • Bohr model was fixed by Louis de Broglie (French physicist - 1924) • The electron is treated mathematically as a wave. • The energy levels are described in terms of the probability of locating the electron in a region of space outside the nucleus. The de Broglie Equation • de Broglie came up with l = h/mv l = wavelength, h = Planck’s constant, m = mass, and v = velocity (m/s) • He decided that since waves can act like particles why couldn’t particles act like waves? • The equation finds the wavelength of a particle. Matter can behave like a Wave - that’s what Einstein said! • But it does not apply to things bigger than an atom • A major league baseball (0.142 kg) has a wavelength of about 10-32 m when moving 30 m/s • Too small to measure • An electron (9.109x10-31kg) at the same speed has a wavelength of 10-3 cm • Big enough to measure. The physics of the very small • Quantum mechanics explains how the very small behaves. • Quantum mechanics is based on probability because… • It is impossible to know exactly the speed and position of a particle at the same time. • The better we know one, the less we know the other. • The act of measuring changes the properties for tiny objects. • This fact is called: Heisenberg Uncertainty Principle • To measure where a electron is, we use light. • But the light moves the electron • And hitting the electron changes the frequency of the light. • See the next slide Before Photon Moving Electron After Photon changes wavelength Electron changes velocity The Quantum Mechanical Model • Austrian physicist Erwin Schrödinger derived a mathematical equation whose solutions describes the probability of finding an electron a certain distance from the nucleus. • Again he treated electrons as waves • Called the solutions quantum numbers Schrodinger is given credit for: The Quantum Mechanical Model • It does have energy levels for electrons. • It does not have orbits. • It has orbitals. The Atomic Orbital: • The electron is found inside a blurry “electron cloud” • A 3-D area where there is a 90 % chance of finding an electron around the nucleus. • They have different energies, sizes and shapes Principal Quantum Number • Given n quantum number • Tells principal energy levels • Tells relative sizes and energies of orbitals • As n increases, orbital size increases & energy level increases • Right now we are up to 7 energy levels with values of 1 – 7 • Always whole numbers • The maximum number of electrons in any principal energy level is 2n2 Energy sublevels • These are given the quantum number l • The number of sublevels in any principal energy level is equal to the value of the principal quantum number • So the 1st principal energy level has 1 sublevel = s-sublevel • The 2nd principal energy level has 2 sublevels = ssublevel and p-sublevel • Sublevels are labeled s, p, d, or f depending on the shape of the orbitals Orbitals • Quantum number, m • These give the orientation of the electron in space • Each individual orbital can only hold a maximum of 2 electrons s-orbitals • There is one s-orbital for every energy level • Spherical shaped • Each s orbital can hold a maximum of 2 electrons • Called the 1s, 2s, 3s, etc.. orbitals. p-orbitals • • • • Start at the second energy level 3 different directions 3 different shapes (dumbell or propeller) Each orbital can hold a maximum of 2 electrons p-orbitals This is a picture of all 3 p-orbitals overlapped d-orbitals • Start at the third energy level • 5 different shapes • Each can hold up to 2 electrons f-orbitals • Start at the fourth energy level • Have seven different shapes • 2 electrons per shape f-orbitals Images J mol Summary # of orbital Max. # of shapes electrons Starts at energy level s 1 2 1 p 3 6 2 d 5 10 3 f 7 14 4