A Dynamic Mathematical Model of Single Nephron Glomerular
A Dynamic Mathematical Model of Single Nephron Glomerular Filtration
Rate in Rat Kidneys
Justin Summerville; Ioannis Sgouralis; Anita T. Layton
Department of Mathematics, Duke University
Abstract
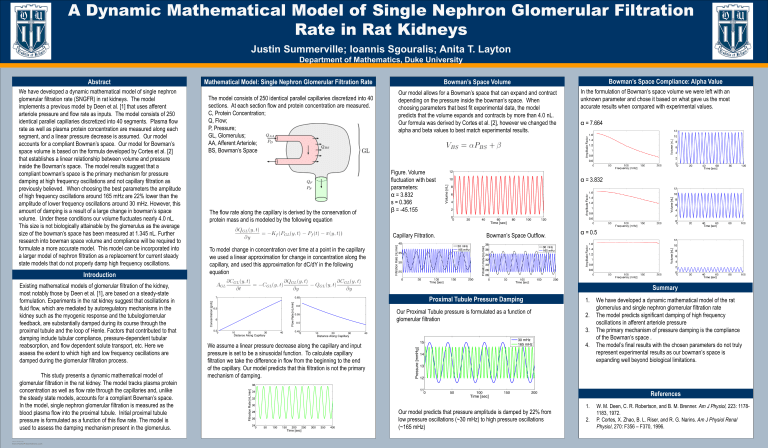
We have developed a dynamic mathematical model of single nephron glomerular filtration rate (SNGFR) in rat kidneys. The model implements a previous model by Deen et al. [1] that uses afferent arteriole pressure and flow rate as inputs. The model consists of 250 identical parallel capillaries discretized into 40 segments. Plasma flow rate as well as plasma protein concentration are measured along each segment, and a linear pressure decrease is assumed. Our model accounts for a compliant Bowman’s space. Our model for Bowman’s space volume is based on the formula developed by Cortes et al. [2] that establishes a linear relationship between volume and pressure inside the Bowman’s space. The model results suggest that a compliant bowman’s space is the primary mechanism for pressure damping at high frequency oscillations and not capillary filtration as previously believed. When choosing the best parameters the amplitude of high frequency oscillations around 165 mHz are 22% lower than the amplitude of lower frequency oscillations around 30 mHz. However, this amount of damping is a result of a large change in bowman’s space volume. Under these conditions our volume fluctuates nearly 4.0 nL.
This size is not biologically attainable by the glomerulus as the average size of the bowman’s space has been measured at 1.345 nL. Further research into bowman space volume and compliance will be required to formulate a more accurate model. This model can be incorporated into a larger model of nephron filtration as a replacement for current steady state models that do not properly damp high frequency oscillations.
Introduction
Existing mathematical models of glomerular filtration of the kidney, most notably those by Deen et al. [1], are based on a steady-state formulation. Experiments in the rat kidney suggest that oscillations in fluid flow, which are mediated by autoregulatory mechanisms in the kidney such as the myogenic response and the tubuloglomerular feedback, are substantially damped during its course through the proximal tubule and the loop of Henle. Factors that contributed to that damping include tubular compliance, pressure-dependent tubular reabsorption, and flow dependent solute transport, etc. Here we assess the extent to which high and low frequency oscillations are damped during the glomerular filtration process.
This study presents a dynamic mathematical model of glomerular filtration in the rat kidney. The model tracks plasma protein concentration as well as flow rate through the capillaries and, unlike the steady state models, accounts for a compliant Bowman’s space.
In the model, single nephron glomerular filtration is measured as the blood plasma flow into the proximal tubule. Initial proximal tubule pressure is formulated as a function of this flow rate. The model is used to assess the damping mechanism present in the glomerulus.
TEMPLATE DESIGN © 2008 www.PosterPresentations.com
Mathematical Model: Single Nephron Glomerular Filtration Rate
The model consists of 250 identical parallel capillaries discretized into 40 sections. At each section flow and protein concentration are measured.
C, Protein Concentration;
Q, Flow;
P, Pressure;
GL, Glomerulus;
AA, Afferent Arteriole;
BS, Bowman’s Space
The flow rate along the capillary is derived by the conservation of protein mass and is modeled by the following equation
To model change in concentration over time at a point in the capillary we used a linear approximation for change in concentration along the capillary, and used this approximation for dC/dY in the following equation
We assume a linear pressure decrease along the capillary and input pressure is set to be a sinusoidal function. To calculate capillary filtration we take the difference in flow from the beginning to the end of the capillary. Our model predicts that this filtration is not the primary mechanism of damping.
Bowman’s Space Volume
Our model allows for a Bowman’s space that can expand and contract depending on the pressure inside the bowman’s space. When choosing parameters that best fit experimental data, the model predicts that the volume expands and contracts by more than 4.0 nL.
Our formula was derived by Cortes et al. [2], however we changed the alpha and beta values to best match experimental results.
Figure. Volume fluctuation with best parameters:
α = 3.832
s = 0.366
β = -45.155
Capillary Filtration. Bowman’s Space Outflow.
Proximal Tubule Pressure Damping
Our Proximal Tubule pressure is formulated as a function of glomerular filtration
Our model predicts that pressure amplitude is damped by 22% from low pressure oscillations (~30 mHz) to high pressure oscillations
(~165 mHz)
Bowman’s Space Compliance: Alpha Value
In the formulation of Bowman’s space volume we were left with an unknown parameter and chose it based on what gave us the most accurate results when compared with experimental values.
α = 7.664
α = 3.832
α = 0.5
Summary
1. We have developed a dynamic mathematical model of the rat glomerulus and single nephron glomerular filtration rate
2. The model predicts significant damping of high frequency oscillations in afferent arteriole pressure
3. The primary mechanism of pressure damping is the compliance of the Bowman’s space .
4. The model’s final results with the chosen parameters do not truly represent experimental results as our bowman’s space is expanding well beyond biological limitations.
References
1.
W. M. Deen, C. R. Robertson, and B. M. Brenner. Am J Physiol, 223: 1178-
1183, 1972.
2.
P. Cortes, X. Zhao, B. L. Riser, and R. G. Narins. Am J Physiol Renal
Physiol, 270: F356 – F370, 1996.