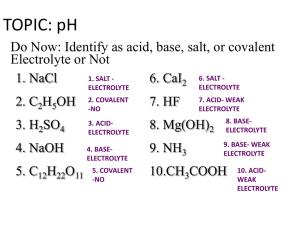
Introduction Questions
advertisement
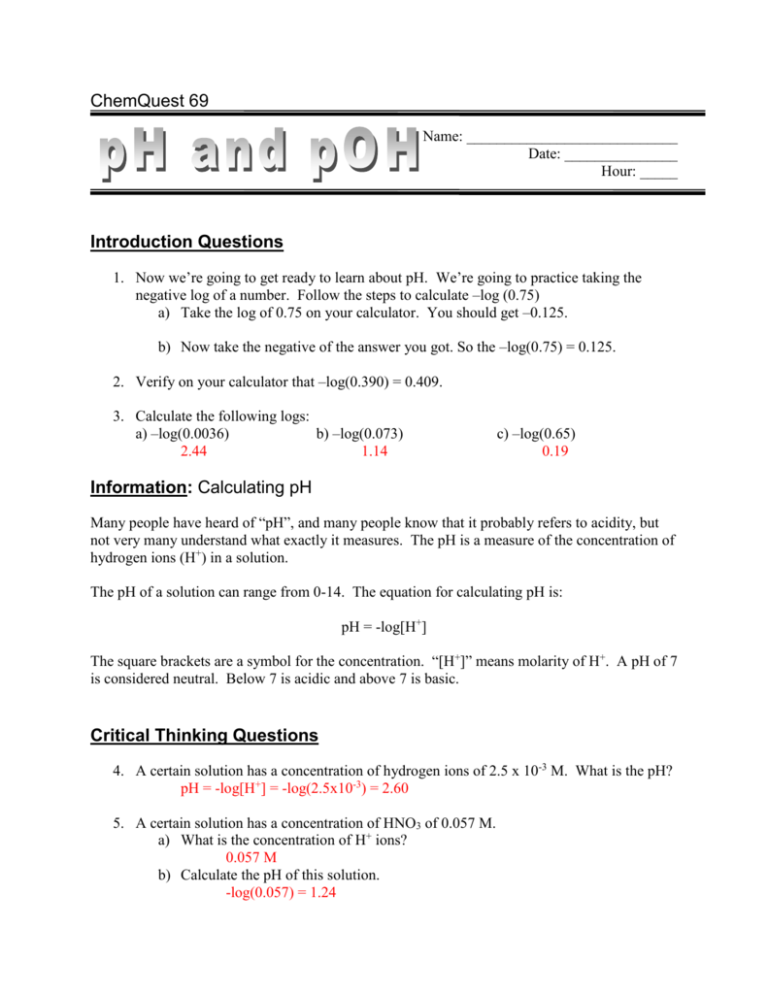
ChemQuest 69 Name: ____________________________ Date: _______________ Hour: _____ Introduction Questions 1. Now we’re going to get ready to learn about pH. We’re going to practice taking the negative log of a number. Follow the steps to calculate –log (0.75) a) Take the log of 0.75 on your calculator. You should get –0.125. b) Now take the negative of the answer you got. So the –log(0.75) = 0.125. 2. Verify on your calculator that –log(0.390) = 0.409. 3. Calculate the following logs: a) –log(0.0036) b) –log(0.073) 2.44 1.14 c) –log(0.65) 0.19 Information: Calculating pH Many people have heard of “pH”, and many people know that it probably refers to acidity, but not very many understand what exactly it measures. The pH is a measure of the concentration of hydrogen ions (H+) in a solution. The pH of a solution can range from 0-14. The equation for calculating pH is: pH = -log[H+] The square brackets are a symbol for the concentration. “[H+]” means molarity of H+. A pH of 7 is considered neutral. Below 7 is acidic and above 7 is basic. Critical Thinking Questions 4. A certain solution has a concentration of hydrogen ions of 2.5 x 10-3 M. What is the pH? pH = -log[H+] = -log(2.5x10-3) = 2.60 5. A certain solution has a concentration of HNO3 of 0.057 M. a) What is the concentration of H+ ions? 0.057 M b) Calculate the pH of this solution. -log(0.057) = 1.24 6. What is the pH of a 0.0085 M solution of HCl? -log(0.0085) = 2.07 Information: pOH As you know, when an acid is placed in water, H+ ions are produced. A base will produce OHions. If pH equals the negative log of the H+ concentration, it should make sense that pOH equals the –log of the OH- ions. pOH = -log[OH-] Critical Thinking Questions 7. The solution from question 4 had a [H+] of 0.0025 M. The very same solution has a concentration of hydroxide ions [OH-] of 4.0 x 10-12 M. What is the pOH of this solution? pOH = -log[OH-] = -log(4.0x10-12) = 11.40 8. Given your answers to questions 4 and 7, which of the following equations is true? A) pH + pOH = 20 B) pH x pOH = 12 C) pH + pOH = 14 D) pH ÷ pOH = 0.85 9. Given the equation you chose from the previous question, calculate the pOH of a solution that has a pH of 4.2. 14 – 4.2 = 9.8 10. Calculate the pH of a 0.025 M solution of Ca(OH)2. You can follow these steps… a) First remember that the OH- concentration is twice the Ca(OH)2 concentration. Calculate the pOH. [OH-] = 0.025x2 = 0.05 M -log(0.05) = 1.3 b) Now use the pOH to calculate the pH (use the equation from question 8). pH = 14 – 1.3 = 12.7 11. Next you can follow these steps to find the H+ concentration from the pH. The equation you use is [H+] = 10-pH. Use the following steps to find the [H+] if the pH of a solution is 2.45. a) First, take the negative of the pH. -2.45 b) Now, use your calculate and hit the “2nd” or “inv” button and then the log button which should be the 10x function. Your answer should be 0.00355. 10-2.45 = 0.0035 M 12. A certain solution has a pH of 5.2. What is the concentration of hydrogen ions [H+]? (Hint: follow the steps from the previous question.) [H+] = 10(-pH) = 10(-5.2) = 6.3x10-6 M 13. a) Find the pOH of the solution from question 12. pOH = 14 – 5.2 = 8.8 a) What is the concentration of hydroxide ions [OH-] in this solution? [OH-] = 10-8.8 = 1.58x10-9 M 14. Given your answers to questions 12 and 13b, which of the following is true: A) [H+] + [OH-] = 1 B) [H+][OH-] = 1x10-14 C) [H+]÷[OH-] = 1 Use what you’ve learned so far to answer the following questions. 15. Calculate the pH of a 0.0078 M solution of Mg(OH)2. 0.0078 x 2 = 0.0156 M pOH = -log(0.0156) = 1.8 pH = 14 – 1.8 = 12.2 16. Consider a 0.00475 M solution of HNO3… a) Calculate the pH. pH = -log(0.00475) = 2.32 b) Calculate the pOH. pOH = 14 – 2.32 = 11.68 c) Calculate the [H+] 0.00475 M d) Calculate the [OH-] 10-11.68 = 2.09x10-12 M 17. A certain solution of HCl has a pH of 1.96. What is the concentration of the acid? [HCl] = [H+] = 10-1.96 = 0.0109 M 18. A certain solution of Ca(OH)2 has a pH of 12.58. What is the concentration of the Ca(OH)2? [OH-] = 10-pOH = 10-1.42 = 0.038 M [Ca(OH)2] = 0.038 ÷ 2 = 0.019 M