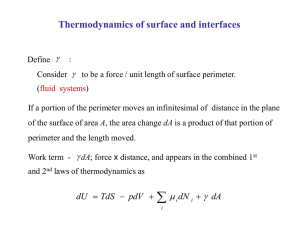
March 20, 2007
advertisement
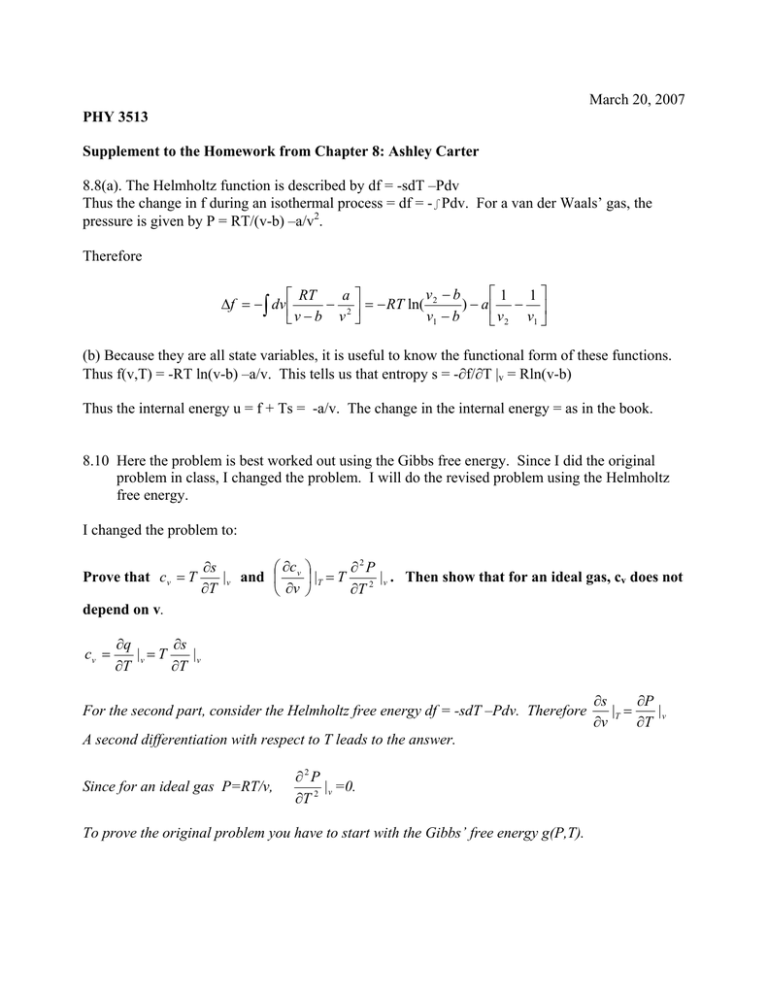
March 20, 2007 PHY 3513 Supplement to the Homework from Chapter 8: Ashley Carter 8.8(a). The Helmholtz function is described by df = -sdT –Pdv Thus the change in f during an isothermal process = df = -∫Pdv. For a van der Waals’ gas, the pressure is given by P = RT/(v-b) –a/v2. Therefore ⎡1 1⎤ v −b a⎤ ⎡ RT ) − a⎢ − ⎥ Δf = − ∫ dv ⎢ − 2 ⎥ = − RT ln( 2 v1 − b ⎣v − b v ⎦ ⎣ v 2 v1 ⎦ (b) Because they are all state variables, it is useful to know the functional form of these functions. Thus f(v,T) = -RT ln(v-b) –a/v. This tells us that entropy s = -∂f/∂T |v = Rln(v-b) Thus the internal energy u = f + Ts = -a/v. The change in the internal energy = as in the book. 8.10 Here the problem is best worked out using the Gibbs free energy. Since I did the original problem in class, I changed the problem. I will do the revised problem using the Helmholtz free energy. I changed the problem to: Prove that cv = T ∂s ⎛ ∂c | v and ⎜ v ∂T ⎝ ∂v ∂2P ⎞ = | T |v . Then show that for an ideal gas, cv does not ⎟ T ∂T 2 ⎠ depend on v. cv = ∂q ∂s |v = T |v ∂T ∂T For the second part, consider the Helmholtz free energy df = -sdT –Pdv. Therefore A second differentiation with respect to T leads to the answer. Since for an ideal gas P=RT/v, ∂2P |v =0. ∂T 2 To prove the original problem you have to start with the Gibbs’ free energy g(P,T). ∂s ∂P |T = |v ∂T ∂v