Lecture 5
advertisement

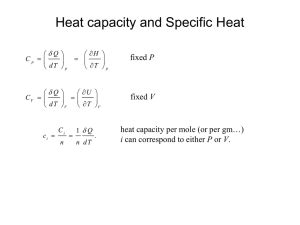
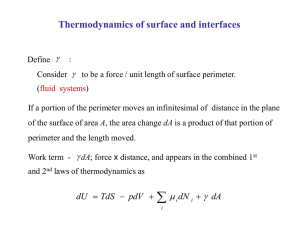
Paperwork • HMWK deadline off by one hour – Everyone get some bonus? • Guest Instructors – Monday – Chapter 20.1-20.3 – Week After Mon & Fri • That’s when the exam is ( 2 weeks) • Move exam back one week, skip one lab? Schedule Short Term • Today – Chapter 19 • Next Week – Monday –Chapter 20.1-20.3 (Guest) – Tuesday – Lab #2 • Quiz#2 [Chapter 18, Labs] – Wed – Ch. 20.4-20.5 – Friday – Practice Problems • Week After – – – – Monday – Ch. 20.6-20.7 (Guest) Tuesday – Lab 3 & Quiz 3 Wed (Was exam) Review 17-20 Friday 21.1-21.3 (Guest) • Then - Chapter 19 • 1st Law of Thermo – Q=DU+W (eq. 19.5) – Q = Heat – W = Work – U = Internal Energy [Any Guesses] • Internal Energy – Sum of all KE [Thermo] – Plus Sum of all interactions [bonds] – Not U as in grav. potential energy Signs & Such • Q=DU+W (eq. 19.5) • Talks about how heat affects a system • What does Q being + mean? – Heat added to system • What happens if DU is positive? – Raise temperature, change state… – Chemical bonds have negative energy – Solid to liquid means DU increases, less neg. • What happens if W is positive? – System does work on its surroundings – Maybe heats up a container, etc… Signs & Such • • • • Q=DU+W (eq. 19.5) Talks about how heat affects a system Q+ heat enters system DU+ Internal energy raised – Bonds broken, temperature increased • W+ Work done on outside world • W- Work done on system from outside Isolated System • • • • • • Q=DU+W System completely isolated from outside What is Q? (say as a function of time) What is W? (time dependence as well) Implications? Isolation can be attained by expansion… Discussion Q18.10 Start Gas # molecules = n0 Temperature = T0 pressure = p0 Volume = V0 Vacuum Discussion Q18.10 “Sudden” Hole in wall Gas Initial State # molecules = n0 Temperature = T0 pressure = p0 Volume = V0 What Happens here? Gas Final State # molecules = ? Temperature = ? pressure = ? Volume = ? Changes in System • Follow equation: Q=DU+W – Look at small changes • • • • dQ = dU + dW dU = dQ – dW dW = pdV [gaseous systems] dU = dQ – pdV [1st law thermo for gas] Types of Changes • • • • • • • Adiabatic (Constant Heat) No heat transfer to/from system Q=0 dU = dQ – dW dU = -dW As a whole: DU = -W For a gas: dU = -pdV Types of Changes • • • • • • • • Isochoric (Constant Volume) No change in volume [Stiff container] dV = 0 pdV = 0 = W dU = dQ As a whole: DU = Q For a gas: DU = Q Usually implies no work done that changes volume • Example: Stirring liquid usually still “isochoric” Types of Changes • • • • • • • • Isobaric (Constant Pressure) No change in volume [Stiff container] p = constant dQ = dU + dW dQ = dU + pdV p is constant of integration, no V dependence Integrate both sides Q = DU + p(DV) Example: Boiling water in an open pot Types of Changes • • • • • • • • • Isothermal (Constant Temperature) No change in Temp [Could add heat though…] T = constant dQ = dU + dW dQ = dU + pdV Complicated pV = nRT p = nRT/V (Ideal Gas) So integration not trivial even for ideal gas Example: Icewater mixture, while both exist Internal Energy of Ideal Gas • Q=DU+W • dQ = dU + dW • Gas: dW = pdV • What does U depend on? – Reminder about U – Measure of internal KE & PE between particles Discussion Q18.10 Start Gas # molecules = n0 Temperature = T0 pressure = p0 Volume = V0 Vacuum Discussion Q18.10 Final State (Again) Q=DU+W Is there work done on gas? Is there heat input? Gas Initial State # molecules = n0 Temperature = T0 pressure = p0 Volume = V0 So what is DU? Gas Final State # molecules = ? Temperature = ? pressure = ? Volume = ? Internal Energy of Ideal Gas • Q=DU+W • What does U depend on? – Temperature – Gas doesn’t change phase (or its not a gas) – Ideal Gas: No interactions between particles – No potential energy (bonding) between particles – Diatomic molecules? Ideal Gas Heat Capacities • • • • • Different for different conditions dQ = nCdT [molar heat capacity] Constant Pressure: CP Constant Volume: CV Constant Temperature: CT – Wouldn’t that mean dQ = 0? • Constant n: Cn – n is not in C, other part of Equation Relationship • CP = CV + R • Derivation in text • Heat capacity larger for isobaric process • • • • • Ratio of heat capacities g = CP/CV = 1.67 (monotomic) g = CP/CV = 1.4 (diatomic) g = CP/CV = 1.3 (“triatomic”) Look at this closer later next week… Reading & Assignments • Chapter 18 assignment – Up today, Due next Friday • Chapter 19 Assignment – Up today, Due in 1.5 weeks • Put up practice problems – Hopefully answers, etc… • Read chapter 19 & sect. 20.1 to 20.3 for Monday Schedule Short Term • Today – Chapter 19 • Next Week – Monday –Chapter 20.1-20.3 (Guest) – Tuesday – Lab #2 • Quiz#2 [Chapter 18, Labs] – Wed – Ch. 20.4-20.5 – Friday – Practice Problems • Week After – – – – Monday – Ch. 20.6-20.7 (Guest) Tuesday – Lab 3 & Quiz 3 Wed (Was exam) Review 17-20 Friday 21.1-21.3 (Guest) • Then -