Chapter 7 The Properties of Matter
advertisement

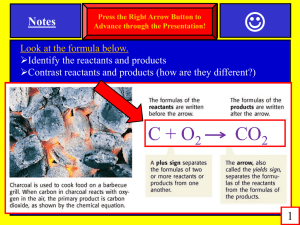
Chapter 7 The Properties of Matter Objectives SPI 0807.9.1 Recognize that all matter consists of atoms SPI 0807.9.7 Apply an equation to determine the density of an object based on its mass and volume SPI 0807.12.4 Distinguish between mass and weight using appropriate measuring instruments and units SPI 0807.12.5 Determine the relationship among the mass of objects, the distance between these objects, and the amount of gravitational attraction 1. 2. 3. 4. 5. Define new vocabulary terms: matter, volume, meniscus, mass, weight, inertia Describe the two properties of all matter Identify the units used to measure volume and mass. Compare mass and weight. Explain how mass affects inertia. Matter • Anything that has mass and takes up space • Everything has matter Volume • Amount of space an object takes up or occupies • All matter takes up space • No two things can occupy the same space at the same time • Measured in three dimensions Measuring Liquid Volume • What tool is used to measure liquid volume? • Graduated cylinder, measuring cups, spoons, beakers • Units: Liter (L) or milliliter (mL) • Meniscus – the curve at the surface of the liquid • Measure volume at the bottom of the meniscus Measuring volume of regular shaped objects • Solid objects expressed in cubic units • Cubic meters (m3)or cubic centimeters (cm3) • Volume = length x width x height • Example: What is the volume of a box that has a length of 5 cm, a width of 1 cm, and a height of 2 cm? • Step 1: Write the equation for volume V=lxwxh • Step 2: Replace the variables with the measurements given to you and solve V = 5 cm x 1 cm x 2 cm = 10 cm3 It’s Your Turn • Calculate the volume for the following: 1. Cube with length of 5 cm 2. Box with height of 4 m, width of 8 cm, and length of 1 cm 3. Book with height of 25 cm, width of 18 cm, and length of 4 cm 4. CD case with 14.2 cm long, 12.4 cm wide, and 1 cm deep Measuring volume of an Irregular Shaped Object • Water Displacement 1. Measure volume of water in graduated cylinder (initial volume) 2. Carefully drop irregular object into grad. cylinder 3. Measure the volume of the water after the water level rises (final volume) 4. Subtract the initial volume from the final volume 5. Units expressed in cm3 • Solid objects are never expressed in L or mL Mass • Amount of matter in an object • What has more matter, an elephant or a rabbit? • The mass of an object is the same no matter where in the universe the object is. • How can you change the amount of matter in a object? Weight • Measure of gravitational force exerted on an object • More mass → greater gravitational force on object → greater weight • Weight changes depending on location in universe • Weigh less on moon than Earth • Weigh more on Jupiter than Earth Mass vs. Weight Mass • Amount of matter • Always constant no matter where located • Measured with balance • Units: kilograms (kg), grams (g), milligrams (mg) • 100g = 1N Weight • Gravitational force on object • Varies depending on location • Measured with spring scale • Units: Newtons (N) Inertia • Tendency of an object to resist change in motion • Object at rest will remain at rest until something causes it to move • Object in motion will stay in motion until something causes it to stop or slow down • Object with more mass is harder to make move and harder to stop Density • Amount of matter in a given volume • Every object has a different density • Used to identify objects • Density is the same no matter the amount of the object • 100 g of silver has a density of 10.50 g/cm3 • 1 g of silver has a density of 10.50 g/cm3 • More dense → sink • Less dense → float • Density of water = 1 g/cm3 • If the density of the object is less than 1 g/cm3, the object will float in water. • If density is greater than 1 g/cm3, the object will sink in water Calculating Density Mass • Density = Volume g g kg • Units: or or 3 cm3 mL m • Example: What is the density of an object whose mass is 25 g and whose volume is 10 cm3? • Step 1: Write the equation for density. D= 𝑚 𝑣 • Step 2: Replace m and V with the measurements given in the problem, and solve. 25 𝑔 D = 10 𝑐𝑚3 = 2.5 g/cm3 Calculating Mass and Volume • Rearrange the density equation to find mass and volume •V= 𝑚 𝐷 •m=DxV Your Turn 1. Find the density of a substance that has a mass of 45 kg and a volume of 40 m3. 2. A block of pine wood has a mass of 120 g. It has a width of 10 cm, length of 10 cm, and a height of 3 cm. What is the density of the wood? Will this block of pine float or sink in a pool of water? Why or why not? 3. What is the density of an object that has a mass of 350 g and a volume of 90 cm3? Will the object float or sink in water? Why or why not? 4. Suppose you have a lead ball whose mass is 454 g. Lead has a density of 10 g/cm3. What is the ball’s volume? 5. What is the mass of a 15 mL sample of mercury? The density of mercury is 13 g/cm3. Objectives • SPI 0807.9.1 Recognize that all matter consists of atoms • SPI 0807.9.8 Interpret the results of an investigation to determine whether a physical or chemical change has occurred 1. 2. 3. 4. 5. 6. 7. 8. Define all new vocabulary terms: physical property, physical change, ductility, malleability, solubility, thermal conductivity, chemical property, chemical change, flammability, reactivity, precipitate. Identify six examples of physical properties of matter. List six examples of physical changes. Explain what happens to matter during a physical change. Explain two examples of chemical properties. List 6 examples of chemical changes. Explain what happens during a chemical change. Distinguish between physical and chemical changes Physical Properties • A characteristic that can be observed or measured without changing the matter’s identity. • Examples: 1. Magnetism 2. Heat conductivity 3. Strength 4. Flexibility 5. Odor 6. Volume 7. Color (sometimes) Physical Properties 1. Thermal conductivity - rate at which substance transfers heat • Foam- poor conductor • Metal - great conductor 2. State – physical form in which a substance exists • Solid, liquid, gas, plasma 3. 4. Density – mass per unit volume Solubility – ability of a substance to dissolve. • Salt in water • Drink mix in water 5. Ductility – ability of a substance to be pulled into a wire • Copper wire 6. Malleability – ability of a substance to be rolled or pounded into this sheets. • Aluminum into foil Physical changes • A change of matter from one form to another without a change in chemical properties. • Do not change the identity of matter involved • Examples: • • • • • • • • Change state Change shape Change color (sometimes) Freezing water Melting a popsicle Tearing paper Dissolving lemonade mix in water Sanding a piece of wood Chemical Properties • The ability to change into new matter that has different properties. 1. Flammability – ability of a substance to burn • Burning wood • Burning a match 2. Reactivity – ability of two or more substances to combine and form one or more new substances. • • • • Reactivity with oxygen – rust (iron and oxygen) Reactivity with acid Reactivity with base Reactivity with light Chemical Changes • A change that occurs when one or more substances change into entirely new substances with different properties. • Changes the identity of the substance • Examples: • • • • • • • Soured milk Rocket blast off Effervescent tablet dissolving in water Statue of Liberty turning green Burning paper Charcoal turning to diamonds Baking a cake Signs of chemical changes • In most chemical changes, more than one sign is present. 1. Change in color (sometimes) 2. Change in odor 3. Production of heat 4. Bubbling 5. Fizzing or foaming 6. Sound or light given off 7. Gas given off 8. Precipitate formed (solid substance formed in solution)