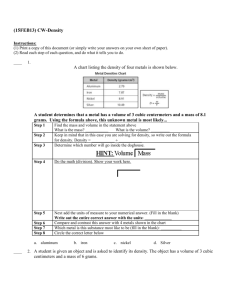
(14JAN16) Study Guide
advertisement
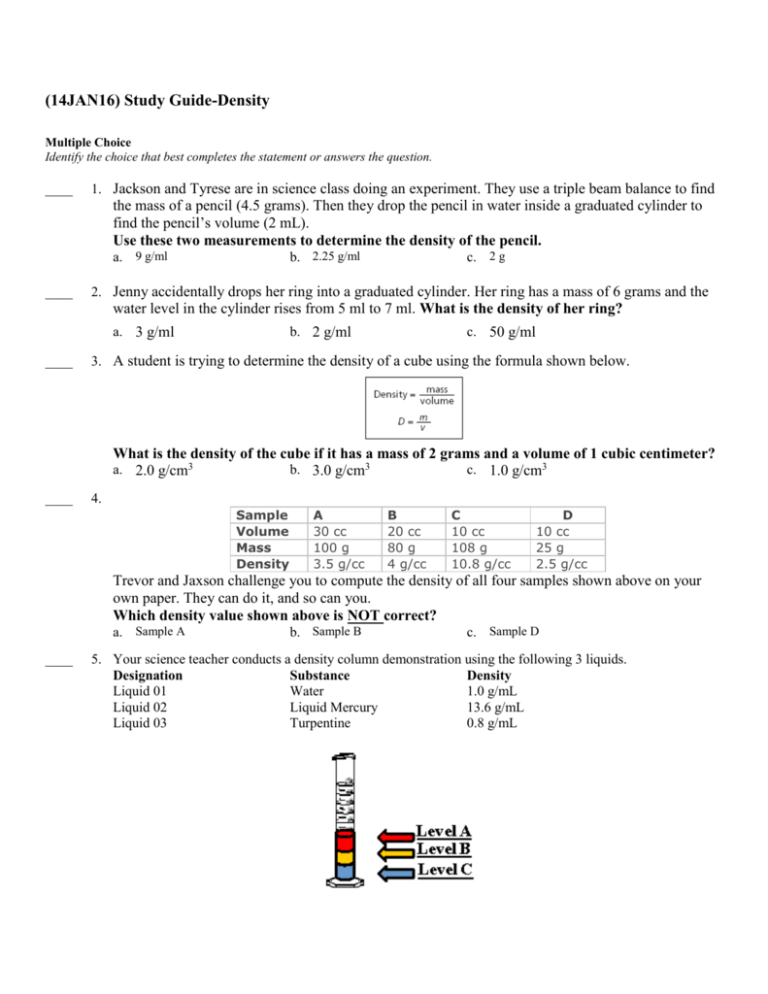
(14JAN16) Study Guide-Density Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Jackson and Tyrese are in science class doing an experiment. They use a triple beam balance to find the mass of a pencil (4.5 grams). Then they drop the pencil in water inside a graduated cylinder to find the pencil’s volume (2 mL). Use these two measurements to determine the density of the pencil. a. 9 g/ml ____ b. 2.25 g/ml c. 2 g 2. Jenny accidentally drops her ring into a graduated cylinder. Her ring has a mass of 6 grams and the water level in the cylinder rises from 5 ml to 7 ml. What is the density of her ring? a. 3 g/ml ____ b. 2 g/ml c. 50 g/ml 3. A student is trying to determine the density of a cube using the formula shown below. What is the density of the cube if it has a mass of 2 grams and a volume of 1 cubic centimeter? a. 2.0 g/cm3 b. 3.0 g/cm3 c. 1.0 g/cm3 ____ 4. Sample Volume Mass Density A 30 cc 100 g 3.5 g/cc B 20 cc 80 g 4 g/cc C 10 cc 108 g 10.8 g/cc D 10 cc 25 g 2.5 g/cc Trevor and Jaxson challenge you to compute the density of all four samples shown above on your own paper. They can do it, and so can you. Which density value shown above is NOT correct? a. Sample A ____ b. Sample B c. Sample D 5. Your science teacher conducts a density column demonstration using the following 3 liquids. Designation Substance Density Liquid 01 Water 1.0 g/mL Liquid 02 Liquid Mercury 13.6 g/mL Liquid 03 Turpentine 0.8 g/mL Select the answer choice below that displays these 3 liquids in the correct order from top to bottom in the graduated cylinder. a. Water, Turpentine, Mercury b. Level C, Level B, Level A c. Turpentine, Water, Mercury ____ ____ 6. Sample Volume A 10 ml B 10 ml C 20 ml D 100 ml The table shows the masses of different samples of liquid. Which sample has the LEAST density? Mass 2g 3g 2g 50 g a. Sample C c. Sample D b. Sample A 7. The equation for density is shown below. Which block has the GREATEST density? a. ____ b. c. 8. A chart listing the density of four metals is shown below. A student determines that a metal has a volume of 3 cubic centermeters and a mass of 8.1 grams. Using the formula above, this unknown metal is most likely...? a. nickel ____ b. silver c. aluminum 9. The density for iron is 7.874 g/cm3. What would be the VOLUME of a piece of iron that has a mass of 196.85g? HINT: This one is not about finding the density. This one is about finding the volume. VMD volume = mass divided by density. a. 25g/cm3 b. 25cm3 c. 0.04cm3 d. 1549.99cm3 ____ 10. Substance Gasoline Diamond Gold Unknown solid Unknown solid Mass 13.6g 2.56g 755g 80g 36g Volume 20cm3 0.8cm3 39.12cm3 50cm3 50cm3 Two part question: (A) What is the density of diamonds? (B) Which has greater density, diamonds or gold? a. 3.2 g/cm3 , diamonds are more dense b. 3.2 g/cm3 , gold is more dense c. 19.3g/cm3, diamonds are more dense Density 0.68g/cm3 ??? 19.3g/cm3 1.6g/cm3 .72g/cm3 (14JAN16) Study Guide-Density Answer Section MULTIPLE CHOICE 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: B A A A C A C C B B PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 1 1 1 1 1 STA: STA: STA: STA: STA: STA: STA: STA: STA: STA: SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7 SPI 0807.9.7