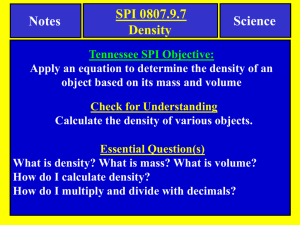
Mass
advertisement
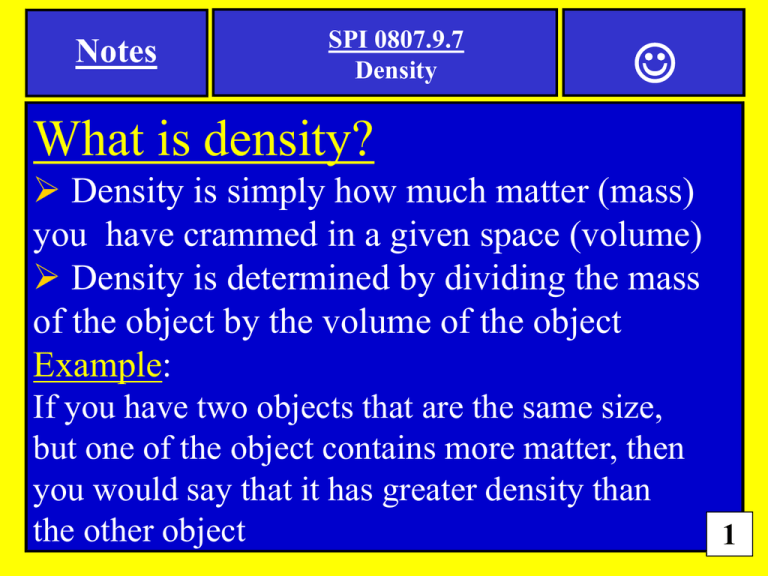
Notes SPI 0807.9.7 Density What is density? Density is simply how much matter (mass) you have crammed in a given space (volume) Density is determined by dividing the mass of the object by the volume of the object Example: If you have two objects that are the same size, but one of the object contains more matter, then you would say that it has greater density than the other object 1 Notes SPI 0807.9.7 Density Feb 7, 2013 1) Please do not use a calculator…work the math on your paper Solve The Three Density Problems Shown Below 2) Use this formula Density=mass divided by volume or D= m/V 1) …density of an object with a mass of 25g a volume of 10cm3? 2) …density of an object with a mass of 45kg a volume of 43m3? 3) …density of an object with a mass of 350g a volume of 95cm3 ? 2 Notes SPI 0807.9.7 Density Solve the density problems below Density=mass divided by volume 1) …density of an object with a mass of 25g a volume of 10cm3? 25g ÷ 10cm3 = 2.5g/cm3 2) …density of an object with a mass of 45kg a volume of 43m3? (round up to the nearest hundredth) 45kg ÷ 43m3 = 1.05kg/m3 3) …density of an object with a mass of 350g a volume of 95cm3 ? (round up to the nearest hundredth) 350g ÷ 95cm3 = 3.68g/cm3 3 Notes SPI 0807.9.7 Density Feb 12, 2013 Designation Substance Density Liquid 01 Water 1.0 g/mL Liquid 02 Liquid Mercury 13.6 g/mL Liquid 03 Turpentine 0.8 g/mL 1) Identify which liquids would occupy levels A, B, and C according to their density. Level A Level B Level C 4 Notes SPI 0807.9.7 Density 2) Which cube below has the GREATEST density? (A) (B) Mass: 19g Volume: 7cm3 Mass: 12g Volume: 3cm3 (C) Mass: 11g Volume: 3cm3 (D) Mass: 23g Volume: 5cm3 5 Notes SPI 0807.9.7 (Density) ANSWERS Designation Substance Density Liquid 01 Water 1.0 g/mL Liquid 02 Liquid Mercury 13.6 g/mL Liquid 03 Turpentine 0.8 g/mL Feb 12, 2013 1) Identify which liquids would occupy levels A, B, and C according to their density. Level A Turpentine (0.8 g/mL) Level B Water (1.0 g/mL) Level C Mercury (13.6 g/mL) 6 Notes SPI 0807.9.7 (Density) ANSWERS 2) Which cube below has the GREATEST density? (B) (A) Mass: 12g Volume: 3cm3 Mass: 19g Volume: 7cm3 4 g/cm3 2.71 g/cm3 (C) (D) Mass: 11g Volume: 3cm3 Mass: 23g Volume: 5cm3 3.6 g/cm3 4.6 g/cm3 7