Notes-LawofConservationofMass
advertisement
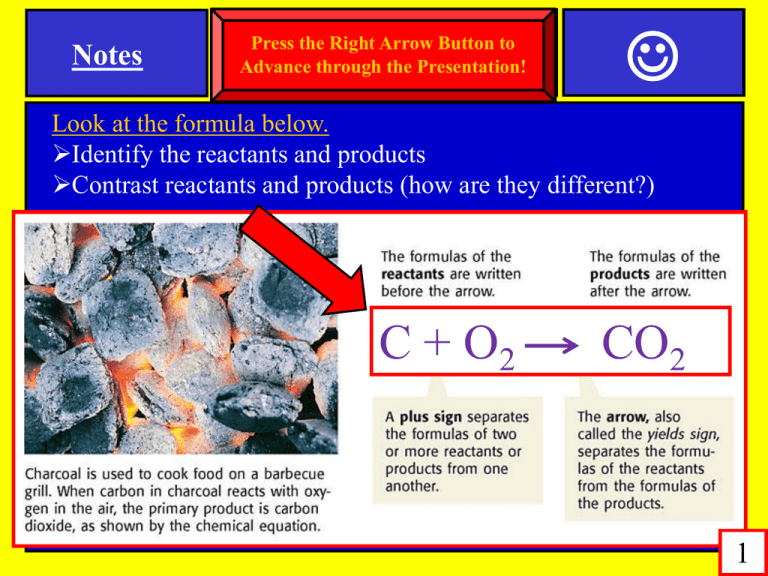
Notes SPI 0807.9.10 & Products Press the Reactants Right Arrow Button to SPIAdvance 0807.9.11through Law of Conservation of Mass the Presentation! Look at the formula below. Identify the reactants and products Contrast reactants and products (how are they different?) C + O2 CO2 1 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Contrast reactants and products (how are they different?) Reactants-are the starting materials in a chemical reactions Remember: the arrow ALWAYS points away from the reactants! C + O2 CO2 2 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass 1) Contrast reactants and products (how are they different?) Products- are the substances formed in a chemical reaction Remember: the arrow ALWAYS points towards the products! C + O2 CO2 3 Review Questions SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Title: LOCOM Review Questions Instructions: (A) Write this assignment title on your paper. (B) Then number your paper from 1 to 17. (C) Write the answers on your paper (D) Review the previous slide(s) to help you (1) ________ and _______ are the 2 parts of all chemical reactions. (2) _________ are the starting materials in a chemical reaction. (3) _________ are the substances you produce in a chemical reaction. (4) In chemical reactions, the arrow points away from the _______. (5) In chemical reactions, the arrow points towards the _________. 4 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass 3 key Topics Chemical symbols Chemical Formulas Chemical Equations 5 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical symbols Examples: H is just a chemical symbol (for the element hydrogen) O is just a chemical symbol (for the element oxygen) Fe is just chemical symbol (for the element iron) 6 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical formulas Chemical symbols combined together = chemical formulas Examples: Put the symbols H (hydrogen) and O (oxygen) together… …and you get the formula for water… H2O Examples: Put the symbols Na (sodium) and Cl (chlorine) together… ...and you get the formula for sodium chloride… NaCl 7 Review Questions SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Title: LOCOM Review Questions Instructions: -Write the answers on your paper -Review the previous slide(s) to help you (6) List 3 key topics that you need to know when learning about chemical reactions. (7) Write any two examples of chemical symbols. (8) Write any two examples of chemical formulas.. 82 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical symbols & Chemical Formulas Very Important!!! symbols & formulas must be written correctly. CO2= one carbon atom, two oxygen atoms CO= one carbon atom, one oxygen atom Co=one cobalt atom 2CO= two carbon atoms, two oxygen atoms 2CO2= two carbon atoms, four oxygen atoms 9 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical symbols & Chemical Formulas QUICK REVIEW! CO2 Is this a chemical symbol or a chemical formula? Is this a mixture or a compound? How many total atoms does it contain? Identify the individual atoms. 10 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical symbols & Chemical Formulas QUICK REVIEW! CO2 Is this a chem symbol or chem formula? It is a chemical formula 11 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical symbols & Chemical Formulas QUICK REVIEW! CO2 Is this a mixture or a compound? It is a compound 12 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical symbols & Chemical Formulas QUICK REVIEW! CO2 How many total atoms does it contain? Three 13 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical symbols & Chemical Formulas QUICK REVIEW! CO2 Identify the individual atoms. = one carbon atom, two oxygen atoms 14 Review Questions SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Title: LOCOM Review Questions Instructions: -Write the answers on your paper -Review the previous slide(s) to help you (9) Is H2O a chemical symbol or chemical formula? (10) The chemical formula CO2 is a compound. How can you tell? (11) H2O contains three total atoms. How many hydrogen atoms does it contain, and how may oxygen atoms does it contain? 15 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Key Topics Chemical Equations Chemical formulas combined together = chemical equations Examples: Equation: 2H2 + O2 2H2O Equation: 2Na + Cl2 2 NaCl 16 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass 2 parts of chemical equations (1) reactants (2) products Reactants: are the things you are combing together…the things you start with in a chemical reaction Products: are the things that you produce in a chemical reaction… the things you end up with Example: 2H2 + 02 2H20 Products (water) Reactants (H & O) 17 Review Questions SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Title: Acid Review Questions Instructions: -Write the answers on your paper -Review the previous slide(s) to help you (12) What do we call the substances created in a chemical reaction? (13) When you see two chemical symbols being added together this is called a chemical __________? (14) Copy down the chemical equation shown below in. Next draw a circle around the reactants. Then draw a rectangle around the products. 2H2 + O2 2H2O 18 2 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Chemical Equations show how chemical reactions occur, and they must obey the LAW OF CONSERVATION OF MASS! The law of conservation of mass: Atoms are never lost or gained in a chemical reaction… … they are just rearranged. Example: Look at the following bad/wrong equation. It DOES NOT obey the law of conservation of mass and is called unbalanced. This equation is wrong. (count the atoms on each side!) H2 + O2 H2O 2 & 2 2&1 Four is not equal to three! 19 Notes SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Balancing Equations: make sure the number of reactants atoms equals the number of products atoms. If they are not equal, you must balance the equation (See below for an example) Same equation as the previous slide, but now I have balanced it (count the atoms on each side) 2H2 + O2 2H2O 4 Hyd + 2 Oxy yields 4 Hyd and 2 Oxy 6 reactant atoms is equal to 6 product atoms! Coefficient=number in front of a chemical symbol or formula (tells you to multiply) Subscript=small number below and to the right of a chemical symbol (tells you the number of atoms) 20 Review Questions SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Title: LOCOM Review Questions Instructions: -Write the answers on your paper -Review the previous slide(s) to help you (15) The law of conservation mass states that atoms are never _____ or gained in a chemical reactions, instead the atoms are merely ____________. (16) Look at the chemical equation shown below. It is unbalanced. How can you tell that this equation is unbalanced? 2H2 + O2 H2O (17) Look at the balanced chemical equation shown below. How can you confirm that it is balanced? 2Na + Cl2 2 NaCl 21 2 Review Questions SPI 0807.9.10 Reactants & Products SPI 0807.9.11 Law of Conservation of Mass Instructions: (A) Click on the study guide link below. (B) Do the study guide until you earn a 100 on it Study Guide Link: See the link under my April 11th website entry (C) Next click on the class work assignment below (D) When complete, click the "Grade & Submit" button. (E) Your grade will be emailed to Mr. Elliott Class work Link: See the link under my April 11th website entry 22 9