Chapter 3
advertisement

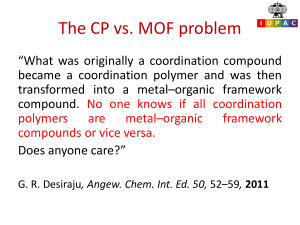
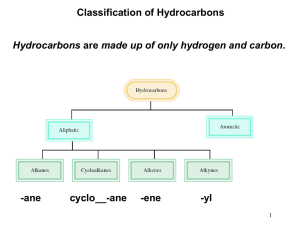
An Introduction to Organic Compounds: Chapter 3 Nomenclature, Physical Properties, and Representation of Structure Chapter 3 1 Contents of Chapter 2 Nomenclature Structures of Alkyl Halides, Alcohols, Ethers, and Amines Physical Properties Conformations of Alkanes Cycloalkanes Chapter 3 2 Counting to Ten in Organic 01 = meth 02 = eth 03 = prop 04 = but 05 = pent 06 = hex 07 = hept 08 = oct 09 = non 10 = dec Mother Enjoys Peanut BUTter PENTagon HEXagon or HEX nut HEPTember (Roman sept is Greek hept) OCTober NONember (Roman nov is Greek non) DECember Chapter 3 3 Alkanes Chapter 3 4 Primary, Secondary, Tertiary A primary carbon has one other C directly bonded to it. A secondary carbon is directly bonded to two other C’s. A tertiary carbon is directly bonded to three other C’s. Multivalent atoms are 1º, 2º, or 3º by bonding to C’s. Univalent atom or group not really 1º, 2º, or 3º on its own - ID depends on type of carbon it is bonded to. Chapter 3 5 Nomenclature of Alkyl Substituents There are four alkyl groups that contain four carbons Chapter 3 6 Nomenclature of Alkyl Substituents The prefix sec- occurs only in sec-butyl Chapter 3 7 Nomenclature of Alkyl Substituents The prefix tert- can be used with butyl or pentyl (also known as amyl) but not with hexyl Chapter 3 8 IUPAC Systematic Nomenclature - Alkanes • • Determine longest continuous chain (i.e. parent hydrocarbon) Cite the name of substituent before the name of the parent hydrocarbon along with the number of the carbon to which it is attached Chapter 3 9 IUPAC Systematic Nomenclature - Alkanes • Number in the direction that gives the lower number for the lowest-numbered substituent. Substituents are listed in alphabetical order – neglecting prefixes such as di- tri- tert- etc. Chapter 3 10 IUPAC Systematic Nomenclature - Alkanes • When both directions yield the same lower number for the lowest numbered substituent, select the direction that yields the lower number for the next lowest numbered substituent Chapter 3 11 IUPAC Systematic Nomenclature - Alkanes • If same substituent numbers are obtained in either direction, number in direction giving lowest number to the first named substituent Chapter 3 12 IUPAC Systematic Nomenclature - Alkanes • If compound has two or more chains of the same length, parent hydrocarbon is chain with greatest number of substituents Chapter 3 13 IUPAC Systematic Nomenclature - Alkanes • • • Names such as sec-butyl and tert-butyl are acceptable, but systematic substituent names are preferable Numbering of the substituent begins with the carbon attached to the parent hydrocarbon This number together with the substituent name is placed inside parentheses Chapter 3 14 Nomenclature of Cycloalkanes Cycloalkanes generally are shown as skeletal structures Chapter 3 15 Nomenclature of Cycloalkanes • • Ring is the parent hydrocarbon unless the alkyl substituent has more carbons; in that case the substituent becomes the parent hydrocarbon If only one substituent, no need to give it a number Chapter 3 16 Nomenclature of Cycloalkanes • If the ring has 2 substituents, list in alphabetical order and give number 1 to first named group Chapter 3 17 Nomenclature of Cycloalkanes • If there is more than one substituent, list in alphabetical order; one substituent is given the position number 1; number either clockwise or counterclockwise - lowest numbers Chapter 3 18 Nomenclature of Alkyl Halides Common name - Name the alkyl group first, followed by the name of the halogen expressed as an -ide name Chapter 3 19 Nomenclature of Alkyl Halides IUPAC name - The halogen is treated as a substituent Chapter 3 20 Nomenclature of Ethers Common name Name(s) of alkyl group(s) listed first followed by the word “ether” Chapter 3 21 Nomenclature of Ethers IUPAC name - The smaller alkyl group is converted to an “alkoxy” name and used as a substituent Chapter 3 22 Nomenclature of Alcohols Common name Name of the Alkyl group followed by the word “alcohol” Chapter 3 23 Nomenclature of Alcohols IUPAC name - The OH group is a site of reactivity (a functional group) Functional group is denoted by the suffix, “ol” CH3OH CH3CH2OH methanol ethanol Chapter 3 24 IUPAC Nomenclature of Alcohols • • • Parent Hydrocarbon is the longest continuous chain that contains the OH Number the chain in direction that gives functional group the lowest number If both a substituent and a functional group are present, the functional group gets the lower number Chapter 3 25 IUPAC Nomenclature of Alcohols • • If the functional group gets the same number when counted from both directions, use direction which gives the substituent the lower number If there is more than one substituent, cite substituents in alphabetical order Chapter 3 26 IUPAC Nomenclature of Alcohols System is summarized as [Substituent] [Parent Hydrocarbon] [Functional Group] Chapter 3 27 Nomenclature of Amines Common name - Name of the Alkyl group(s) (in alphabetical order) followed by the syllable “amine” The whole name is a single word methylamine methylpropylamine Chapter 3 28 Nomenclature of Amines IUPAC name - The NH2 group is a site of reactivity (a functional group) Functional group is denoted by the suffix, “amine” Final “e” of longest alkane group replaced by suffix “amine” (don’t run vowels together) CH3CH2CH2CH2NH2 1-butanamine butan-1-amine Chapter 3 29 IUPAC Nomenclature of Amines • Find the longest chain bonded to the nitrogen • Final “e” is replaced with “amine” • Number the carbon to which nitrogen is bonded • Number any substituents on the alkyl chain • Use italicized N- for each additional substituent(s) on the nitrogen Chapter 3 30 Properties of Alkyl Halides, Alcohols, Ethers, & Amines For alkanes, there are only induced dipole-induced dipole interactions (also known as van der Waals forces or London forces) van der Waals forces are a function of surface area Chapter 3 31 Induced Dipole-Induced Dipole Interactions Chapter 3 32 Hydrogen Bonding: Strong Dipole-Dipole Interactions Chapter 3 33 Dipole-dipole Interactions Particularly important for alcohols and amines Ethers and alkyl halides have dipole moments, but their intermolecular attractions are not as strong as hydrogen bonds Chapter 3 34 Comparative Boiling Points Chapter 3 35 Solubility The more carbons that are present, the less soluble an organic compound is in water Chapter 3 36 Conformations of Alkanes: Rotation About C-C Single Bonds Chapter 3 37 Drawing Cyclohexane in the Chair Conformation Chapter 3 38 Interconversion of Cyclohexane Conformations As a result of simultaneous rotation about all C-C bonds, a chair conformation of cyclohexane can interconvert to another chair conformation by a ring-flip In the process, equatorial bonds become axial and vice versa Chapter 3 39 Monosubstituted Cyclohexanes When there is one substituent on the cyclohexane ring, the two chair conformations are no longer equivalent Chapter 3 40 Conformations of 1,4Disubstituted Cyclohexanes two methyl groups on same side of ring two methyl groups on opposite sides of ring H CH3 H cis-1,4-dimethylcyclohexane CH3 H3C CH3 H H trans-1,4-dimethylcyclohexane Chapter 3 41 Conformations of 1,4Disubstituted Cyclohexanes The cis isomer must have one substituent in an axial position and one in an equatorial position H H ring-flip CH3 H CH3 H3C equatorial axial H CH3 axial cis-1,4-dimethylcyclohexane Chapter 3 42 Conformations of 1,4Disubstituted Cyclohexanes The trans isomer has both substituents in either the equatorial or in the axial positions axial CH3 H ring-flip H CH3 H3C H equatorial equatorial CH3 H much more stable much less stable trans-1,4-dimethylcyclohexane Chapter 3 axial 43 Conformations of cis-1,3Disubstituted Cyclohexanes A cis-1,3-disubstituted cyclohexane can exist in one of two conformations H ring-flip H H CH3 H3C C H3C CH3 CH3 H C CH3 CH3 CH3 much more stable much less stable cis-1-tert-butyl-3-methylcyclohexane Chapter 3 44 Conformations of trans-1,3Disubstituted Cyclohexanes Both conformers of trans-1-tert-butyl-3methylcyclohexane have one substituent in an axial position and one in an equatorial position Chapter 3 45