Alkenes and Alkynes Ch#3
advertisement

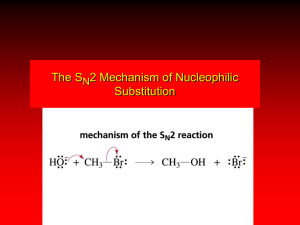
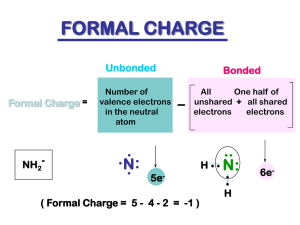
Alkenes and Alkynes Chapter #3 Alkene Introduction • Hydrocarbon with carbon-carbon double bonds • Sometimes called olefins, “oil-forming gas” • General formula CnH2n n≥2 • Examples n=2 C2H4 Common Names Usually used for small molecules. Examples: CH2=CH2 ethylene CH2=CH-CH3 propylene CH3 CH2=C-CH3 isobutylene Vinyl carbons are the carbons sharing a double bond in blue Vinyl hydrogens are the hydrogens bonded to vinyl carbons in red IUPAC Nomenclature • Parent is longest chain containing the double or triple bond. • -ane changes to –ene (or -diene, -triene) for double bonds, or –yne (or –diyne, -triyne). • Number the chain so that the double bond, or triple bond has the lowest possible number. • In a ring, the double bond is assumed to be between carbon 1 and carbon 2. Name These Alkenes CH2 CH CH2 CH3 CHCH2CH3 H3C CH3 C CH CH3 CH3 CH3 Name These Alkenes CH2 CH CH2 1-butene CH3 C CH CH3 CH3 CH3 CH3 CHCH2CH3 H3C Name These Alkenes CH2 CH CH2 CH3 1-butene CH3 C CH CH3 CH3 2-methyl-2-butene CH3 CHCH2CH3 H3C Name These Alkenes CH2 CH CH2 CH3 1-butene CH3 C CH CH3 CH3 2-methyl-2-butene CH3 3-methylcyclopentene CHCH2CH3 H3C Name These Alkenes CH2 CH CH2 CH3 1-butene CHCH2CH3 H3C 2-sec-butyl-1,3-cyclohexadiene CH3 C CH CH3 CH3 2-methyl-2-butene CH3 3-methylcyclopentene Name These Alkenes CH2 CH CH2 CH3 1-butene CHCH2CH3 H3C 2-sec-butyl-1,3-cyclohexadiene CH3 C CH CH3 CH3 2-methyl-2-butene CH3 3-methylcyclopentene 3-n-propyl-1-heptene Alkene Substituents = CH2 - CH = CH2 methylene vinyl Name = ? - CH2 - CH = CH2 allyl - CH2 - CH = CH2 allyl Alkene Substituents = CH2 - CH = CH2 methylene vinyl Name = Methylenecyclohexane - CH2 - CH = CH2 allyl Name = - CH2 - CH = CH2 allyl Alkene Substituents = CH2 - CH = CH2 methylene vinyl Name = Methylenecyclohexane - CH2 - CH = CH2 allyl Name = vinylcyclohexane Alkyne Common Names • Acetylene is the common name for the two carbon alkyne. • To give common names to alkynes having more than two carbons, give alkyl names to the carbon groups attached to the vinyl carbons followed by acetylene. Alkyne Examples Alkyne Examples Isopropyl methyl acetylene Alkyne Examples Isopropyl methyl acetylene sec-butyl Cyclopropyl acetylene Cis-trans Isomerism • Similar groups on same side of double bond, alkene is cis. • Similar groups on opposite sides of double bond, alkene is trans. • Cycloalkenes are assumed to be cis. • Trans cycloalkenes are not stable unless the ring has at least 8 carbons. Name these: H CH3 C C CH3CH2 H Name these: H CH3 C C CH3CH2 trans-2-pentene H Name these: H CH3 Br C C C C CH3CH2 trans-2-pentene Br H H H Name these: H CH3 Br C C CH3CH2 trans-2-pentene Br C C H H H cis-1,2-dibromoethene Which of the following show cis/trans isomers? a. 1-pentene b. 2-pentene c. 1-chloro-1-pentene d. 2-chloro-1-pentene e. 2-chloro-2-pentene Solution to the Question Solution to the Question Which of the following show cis/trans isomers? a. 1-pentene-No b. 2-pentene- Yes c. 1-chloro-1-pentene- Yes d. 2-chloro-1-pentene- No e. 2-chloro-2-pentene- yes E-Z Nomenclature • Use the Cahn-Ingold-Prelog rules to assign priorities to groups attached to each carbon in the double bond. Highest priority is #1 and is the element with the largest atomic number. • If high priority groups are on the same side, the name is Z (for zusammen). • If high priority groups are on opposite sides, the name is E (for entgegen). Example, E-Z 1 H3C Cl C C H 2Z 1 H CH2 2 2 Cl 2 1 CH CH3 C C H 2 1 5E Example, E-Z 1 H3C Cl C C H 2Z 1 H CH2 2 2 Cl 2 1 CH CH3 C C H 2 1 5E 3,7-dichloro-(2Z, 5E)-2,5-octadiene Physical Properties • Low boiling points, increasing with mass. • Branched alkenes have lower boiling points. • Less dense than water. •Nonpolar (Hydrophobic) Alkene Synthesis Elimination Reactions: • Dehydrohalogenation (-HX) • Dehydration of alcohols (-H2O) Examples: Cl + + NaCl + HOH + NaOH major minor OH H + H2O minor major Zaitsev’s rule: The major product contains the most substituted double bond Alkene Reactions I. Addition Reactions a. Hydration H O-H H+ C=C + H-O-H H H Catalyst + H-H C-C Alkane c. Halogenation C=C + X-X X = Cl, Br, I Follows Markovnikov’s Rule Alcohol b. Hydrogenation C=C C-C X X C-C Dihalide Catalyst = Ni, Pt, Pd Regiospecificity Markovnikov’s Rule: The proton (H+) of an acid adds to the carbon in the double bond that already has the most H’s. “Rich get richer.” H O-H Examples: H H H+ + H-O-H C=C H CH3 H H C=C H + H-Cl CH3 H C-C H H CH3 Major Products H Cl H C-C H H CH3 Alkene Reactions (2) I. Addition Reactions (cont.) d. Hydrohalogenation C=C + H-X H X Follows Markovnikov’s Rule C-C Alkyl halide e. Glycol Formation C=C + H-O-O-H H-O O-H C-C Glycol Alkene Reactions Step 1: Pi electrons attack the electrophile. Step 2: Nucleophile attacks the carbocation Terpenes • Composed of 5-carbon isopentyl groups. • Isolated from plants’ essential oils. • C:H ratio of 5:8, or close to that. • Pleasant taste or fragrant aroma. • Examples: Myrcene (From bay or myrcia plants) α-Pinene (From pine trees) Β-Selinene (From celery) Menthol (From peppermint oil) Camphor (From evergreen trees) R-Carvone (From spearmint) Classification • Terpenes are classified by the number of carbons they contain, in groups of 10. • A monoterpene has 10 C’s, 2 isoprenes. • A diterpene has 20 C’s, 4 isoprenes. • A sesquiterpene has 15 C’s, 3 isoprenes. Terpenes head tail head head tail tail 2-methyl-1,3-butadiene Isoprene OH tail head Geraniol (roses) Head to tail link of two isoprenes Called diterpene head Menthol (pepermint) Head to tail link of two isoprenes another diterpene Structure of Terpenes Two or more isoprene units, 2-methyl-1,3-butadiene with some modification of the double bonds. myrcene, from bay leaves ALKENE REVIEW Describe the geometry around the carbon–carbon double bond. a. Tetrahedral b.Trigonal pyramidal c. Trigonal planar d.Bent e.Linear Answer a. Tetrahedral b.Trigonal pyramidal c. Trigonal planar d.Bent e.Linear Give the formula for an alkene. a. CnH2n-4 b.CnH2n-2 c. CnH2n d.CnH2n+2 e.CnH2n+4 Answer a. CnH2n-4 b.CnH2n-2 c. CnH2n d.CnH2n+2 e.CnH2n+4 Name CH3CH=CHCH=CH2. a. 2,4-butadiene b.1,3-butadiene c. 2,4-pentadiene d.1,3-pentadiene e.1,4-pentadiene Answer a. 2,4-butadiene b.1,3-butadiene c. 2,4-pentadiene d.1,3-pentadiene e.1,4-pentadiene Calculate the unsaturation number for C6H10BrCl. a. 0 b.1 c. 2 d.3 Answer a. 0 b.1 c. 2 d.3 U = 0.5 [2(6) + 2 – (12)] = 1 Name H3C . C CH3 C H a. b. c. d. Trans-2-pentene Cis-2-pentene Trans-3-methyl-2-pentene Cis-3-methyl-2-pentene CH2CH3 Name H3C CH3 C H a. b. c. d. C CH2CH3 Trans-2-pentene Cis-2-pentene Trans-3-methyl-2-pentene Cis-3-methyl-2-pentene Name H3C CH3 C H a. b. c. d. e. C CH2CH3 E-2-pentene Z-2-pentene E-3-methyl-2-pentene Z-3-methyl-2-pentene Z-2-methyl-2-pentene Name H3C CH3 C H a. b. c. d. e. C CH2CH3 E-2-pentene Z-2-pentene E-3-methyl-2-pentene Z-3-methyl-2-pentene Z-2-methyl-2-pentene H H Cl2 C H a. ClCH2CH2Cl b.ClCH=CHCl c. CH2=CH2 d.CH2=CHCl C H NaOH Answer a. ClCH2CH2Cl b.ClCH=CHCl c. CH2=CH2 d.CH2=CHCl Chlorine is added across the double bond, then HCl is lost. H CH3 C H2O C catalyst H a. (CH3)2CHOH b.CH3CH2CH2OH c. HOCH2CH2CH2OH d.CH3CH(OH)CH2OH H Answer a. (CH3)2CHOH b.CH3CH2CH2OH c. HOCH2CH2CH2OH d.CH3CH(OH)CH2OH Water adds by Markovnikov’s orientation across the double bond. Identify the product formed from the polymerization of tetrafluoroethylene. a. Polypropylene b.Poly(vinyl chloride), (PVC) c. Polyethylene d.Poly(tetrafluoroethylene), Teflon Answer a. Polypropylene b.Poly(vinyl chloride), (PVC) c. Polyethylene d.Poly(tetrafluoroethylene), Teflon Teflon is formed from the polymerization of tetrafluoroethylene. H3C CH3 C H2 C Pd H a. CH3CCCH3 b.CH2=CHCH=CH2 c. CH3CH=CHCH3 d.CH3CH2CH2CH3 H Answer a. CH3CCCH3 b.CH2=CHCH=CH2 c. CH3CH=CHCH3 d.CH3CH2CH2CH3 Hydrogen adds across the double bond to form an alkane. H3C H C OH a. (CH3)2CHOSO3H b.CH3CH=CH2 c. (CH3)2C=O d.CH3CH2COOH H2SO4 CH3 heat 7.15 Answer a. (CH3)2CHOSO3H b.CH3CH=CH2 c. (CH3)2C=O d.CH3CH2COOH Acid dehydrates alcohols to form alkenes. Give the products from the catalytic cracking of alkanes. a. Alkanes b.Alkenes c. Alkynes d.Alkanes + alkenes e.Alkanes + alkynes Answer a. Alkanes b.Alkenes c. Alkynes d.Alkanes + alkenes e.Alkanes + alkynes Give the products from the dehydrogenation of alkanes. a. Alkanes b.Alkenes c. Alkynes d.Alkanes + alkenes e.Alkanes + alkynes Give the products from the dehydrogenation of alkanes. a. Alkanes b.Alkenes c. Alkynes d.Alkanes + alkenes e.Alkanes + alkynes End Chapter #3