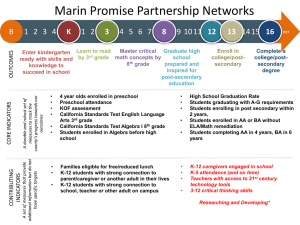
Early molecular prediction of
response to TKI
David Marin,
Imperial College London
I do not believe that we have been making the best of
molecular monitoring.
• Molecular monitoring is normally used to assess responses like
MR3 and MR4.5 or CMR that are achieved late on therapy (while
cytogenetics are used to assess responses early on therapy)
• The current definitions of molecular response are partially arbitrary
and with very little prognostic relevance
One example of this is MR3 (MMR)
PFS is similar in patients with CCyR regardless of the
depth of molecular response
18 months
1.0
0.9
0.8
Probability of PFS
CCyR with no MMR, n=91
0.7
CCyR with MMR, n= 41
0.6
p= 0.4
0.5
0.4
0.3
0.2
0.1
0.0
0
6
12
18
24
30
36
42
48
54
60
Months from starting imatinib therapy
Marin et al, Blood 2008
PFS similar in patients with CCyR regardless of
depth of molecular response
Druker BJ, et al. NEJM, 2006;355(25):2408-17.
PFS is similar in patients with CCyR regardless
of depth of molecular response
Kantarjian HM, et al. Blood. 2006;108:1835-1840.
Maybe we have the concept of MMR wrong?
The real question is:
What is the additional reduction in the
transcript level that confers survival
advantage in patients already in
CCyR ?
Patient characteristics (n=282)
Characteristics
Age
Median-yr
Range-yr
46.3
13-86.4
Sex- no.(%)
Male
Female
157 (55.7)
125 (44.3)
Sokal risk group - no.(%)
Low
Intermediate
High
88 (31.8)
111 (40.1)
78 (28.1)
Interval since diagnosis – (months)
Median
Range
1.5
0-6
Chromosomal abnormalities in addition to the Ph
chromosome
no. (%)
16 (6.0)
Splenomegaly
n (%)
Spleen size ≥10cm below the costal margin, n (%)
186 (66.4)
75 (26.8)
Marin et al, JCO 2011
Outcome in 282 patients treated with imatinib
first line in Hammersmith Hospital
94%
6% death non CML
84%
10% death because the CML
77%
7% alive but not in CCyR
29% in CCyR but not on imatinib
48%
48% in CCyR on imatinib
Marin et al, JCO 2011
Using appropriate statistical methodology we can identify the cut
off in the 12 month transcript level for patients in CCyR that
predicts for OS with the maximal sensitivity and specify.
BCR-ABL/ABL= 0.53%
Marin et al, JCO 2011
166 out of 282 patients who received imatinib as first
line therapy were in CCyR at 12 months
Transcript
level
>0.1%
<0.1%
125
41
OS
(%)
EFS
(%)
p=0.5
94.2
96.3
p=0.08
80.4
93.7
Marin et al, JCO 2011
166 out of 282 patients who received imatinib as first
line therapy were in CCyR at 12 months
Transcript
level
>0.1%
<0.1%
>0.53%
<0.53%
OS
(%)
EFS
(%)
125
41
p=0.5
94.2
96.3
p=0.08
80.4
93.7
20
146
p=0.01
81.5
94.4
p<0.0001
29.5
74.3
Marin et al, JCO 2011
Can we define robust molecular levels
at 3, 6 or 12 month that have
prognostic value?
Patients outcome according to
the transcript level measured at 3 month
Outcome
OS
PFS
EFS
CCyR
MR3
CMR
cutoff
(%)
≤9.84
>9.84
≤9.54
>9.54
≤9.84
>9.84
≤8.58
>8.58
≤2.81
>2.81
≤0.61
>0.61
Number of
patients at
risk
Eight years
probability of the
outcome
211
68
p<0.0001
93.3
56.9
208
71
p<0.0001
92.8
57.0
211
66
p <0.0001
65.1
6.9
169
79
p<0.0001
99.4
21.7
141
137
p<0.0001
82.5
21.1
57
222
p<0.0001
84.7
1.5
Marin et al, JCO 2011
Survival for 282 patients treated with imatinib first line
at the Hammersmith Hospital according to molecular
response achieved at 3 months
Probability of survival
BCR-ABL/ABL<9.8% OS= 93.3%
BCR-ABL/ABL>9.8% OS= 54%
p<0.0001
Time from onset of imatinib therapy (years)
Marin et al, JCO 2011
8-year cumulative incidence of CMR on imatinib therapy
according to the BCR-ABL transcript level at 3 months.
Cumulative incidence of CMR
3-month transcript ratio ≤0.61% (n=57), 8-year CI of CMR of 84.7%,
p<0.0001
3-month transcript ratio >0.61% (n=222), 8-years CI of CMR of 1.5%
Time from onset of therapy (years)
Marin et al, JCO 2011
Patient outcome according to BCR-ABL transcript
level measured at 6 and 12 month
6 month
12 month
Outcome
cut-off
(%)
Number
of
patients
at risk
OS
≤1.67
>1.67
187
87
p<0.0001
93.7
74.7
PFS
EFS
Eight years
probability of
the outcome
PFS
≤1.73
>1.73
188
86
p<0.0001
92.8
68.9
EFS
≤1.67
>1.67
186
87
p<0.0001
70.7
18.3
98
66
p<0.0001
92.0
24.7
CCyR
≤2.70
>2.70
MMR
≤0.73
>0.73
136
123
p<0.0001
81.6
20.4
CMR
≤0.21
>0.21
73
197
p<0.0001
42.7
6.1
Outcome
OS
MMR
CMR
cut-off
(%)
Number
of
patients
at risk
≤0.53
>0.53
164
93
p<0.0001
95.4
74.7
≤0.53
>0.53
164
92
p<0.0001
94.9
73.1
≤ 0.57
>0.57
168
78
p<0.0001
82.1
41.4
90
114
p<0.0001
81.6
20.4
59
182
p<0.0001
52.1
4.1
≤0.22
>0.22
≤0.036
>0.036
Eight years
probability of the
outcome
Marin et al, JCO 2011
These cut-offs can be used in other centres when the
transcript level is expressed on the international scale
Transcript ratio
We have validated our findings
using 95 patients treated with
imatinib first line at the Royal
Liverpool University Hospital
3 month
≤9.84%
>9.84%
6 month
≤1.67%
>1.67%
12 month
≤0.53%
>0.53%
Marin et al, JCO 2011
n
OS
63
32
p=0.003
98.3
69.1
54
40
p=0.009
98.1
7.2
46
37
p=0.01
98.0
74.4
The predictive value of the transcript level measured at
3 months is not affected by imatinib dose reductions
n
3 months
BCR-ABL/ABL
ratio
CCyR
(RR)
c-CCyRS
(RR)
OS
(RR)
186
5.8%
1
1
1
Non-haematological
toxicity
23
7.1%
p=0.8
0.94
p=0.6
1.12
p=0.5
0.97
p=0.6
Haematological
toxicity
70
8.9%
p<0.001
0.44
p<0.001
0.53
p<0.001
2.3
p<0.001
No discontinuation
Marin et al, JCO 2011
What is the best moment to identify
the poor risk patients?
3 months is the optimal time point to predict
a patient’s outcome
Off
Imatinib
high transcript level at 3 month
BCR-ABL1/ABL1 (log)
low transcript level at 3 month
CMR
Marin et al, JCO 2011
Results of transcript levels at 3 and 6
months are generally consistent
66% (L/L)
(85%)
77%
6 month
(1.67%)
11% (L/H)
3 month
milestone
9.84%
2% (H/L)
23%
6 month
(1.67%)
21% (H/H)
(91%)
8 year CI of CCyR
Low/Low (L/L)
Low/ high (L/H)
(L/L) Low at 3 and low at 6 months
p<0.001
P<0.001
(L/H) Low at 3 and high at 6 months
(H/L) High at 3 and low at 6 months
(H/H) High at 3 and high at 6 months
High/Low (H/L)
P=0.09
High/High (H/H)
8 year probability of OS
93.5 % (L/L)
92.4 % (L/H)
83.3 % (H/L)
P<0.001
55.6 % (H/H)
(L/L) Low at 3 and low at 6 months
(L/H) Low at 3 and high at 6 months
(H/L) High at 3 and low at 6 months
(H/H) High at 3 and high at 6 months
The same principle applies
to patients treated with 2G-TKI
after imatinib failure
OS and c-CCyRS in 107 patients treated with
dasatinib or nilotinib after imatinib failure
3 months BCR-ABL/ABL <10%, n=77
3 months BCR-ABL/ABL >10%, n=30
Probability of OS
Probability of c-CCyRS
p=0.001
p=0.02
Time (years)
Time (years)
Milojkovic et al, Blood 2012
What about first line 2G-TKI?
SPIRIT 2: Study Design
Arm A
Imatinib 400
Chronic phase CML
within 3 months of diagnosis
R
Arm B
Dasatinib 100
Randomised open label study
Dasatinib patient characteristics
N=142
Sex
Male, n(%)
Female, N(%)
79 (55.6)
63 (44.4)
Age
Median (range)
54.5 (18-82)
Sokal risk group
Low, n(%)
Intermediate, n(%)
High n(%)
35 (29.9)
51 (43.6)
31 (26.5)
EUTOS risk group
Low, n(%)
High, n(%)
86 (83.5)
17(16.5)
Marin et al, Blood 2012
24 month cumulative incidences of cytogenetic
and molecular responses
Cumulative incidence of response
CCyR = 91.5%
MR3 = 70.1%
MR4.5 = 39.1%
CMR = 6.5%
Time from onset of therapy (months)
Marin et al, Blood 2012
The BCR-ABL1 transcript levels at 3 month strongly
predict for the 2 year CI of CCyR, MR3 and, MR4.5
MR4.5
BCR-ABL/ABL >10%, n=11
p<0.001
Time from onset of therapy (months)
Cumulative incidence of MR4.5
BCR-ABL/ABL ≤10%, n=117
Cumulative incidence of MR4.5
Cumulative incidence of CCyR
CCyR
p<0.001
BCR-ABL/ABL ≤10%, n=117
BCR-ABL/ABL >10%, n=11
Time from onset of therapy (months)
Marin et al, Blood 2012
….. but the optimal cut-offs may turn out to be lower
MR4.5
CCyR
BCR-ABL/ABL ≤2.26%, n=88
BCR-ABL/ABL >2.26%, n=40
Cumulative incidence of MR4.5
Cumulative incidence of CCyR
p<0.0001
BCR-ABL/ABL ≤0.57%, n=62
BCR-ABL/ABL >0.57%, n=66
p<0.0001
Time from onset of therapy (months)
Time from onset of therapy (months)
Marin et al, Blood 2012
Thanks to:
Dragana Milojkovic
&
John Goldman