File
advertisement

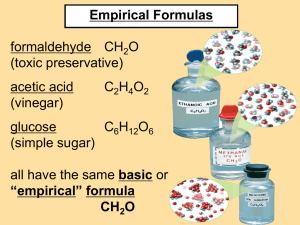
Catalyst 1. To fill up a cup with water, let’s say you need 5 billion molecules of water. How many atoms of hydrogen is that? How many atoms of oxygen? 2. When you heat up food in the microwave, how do you know how long to set it for? What is balanced in a chemical equation? Three things are balanced in a chemical equation: 1. Atoms 2. Mass 3. Charge Balancing Chemical Equations Atoms must be conserved! For now, you will only be concerned with balancing atoms. Remember, the number of atoms of each element on the reactants side must equal the number of atoms of each element on the products side! Counting Atoms • How many atoms of each element are in the following molecules? 1. 2. 3. 4. 5. H2SO4 CaOH2 NaCl (NH3)3PO4 3H2O Answers 1. 2 hydrogen, 1 sulfur, 4 oxygen 2. 1 calcium, 1 oxygen, 2 hydrogen 3. 1 sodium, 1 chlorine 4. 3 nitrogen, 9 hydrogen, 1 phosphorus, 4 oxygen 5. 6 hydrogen, 3 oxygen Is this equation balanced? • NaCl + H2O NaOH + Cl2 • left side has 1Na, 1Cl, 2H, and 1O • right side has 1Na, 1O, 1H, and 2Cl • NO! It is not balanced! Is this equation balanced? • HCl + NaOH NaCl+ H2O • left side has 2H, 1Cl, 1Na, and 1O • right side has 1Na, 1Cl, 2H, and 1O • Yes! It is balanced! Is this equation balanced? • Ca + H2O Ca(OH)2 + H2 • left side has 1Ca, 2H, 1O • right side has 1Ca, 2O, 4H • No! It is not balanced! • How can we make it balance? Ca + H2O Ca(OH)2 + H2 We need more H’s, but we can’t add just the H atom, we have to add the entire molecule the H is attached to: Ca + H2O Ca(OH)2 + H2 H2O Count the boxes. These are the coefficients: 1 Ca + 2 H2O 1 Ca(OH)2 + 1 H2 1 Ca + 2 H2O 1 Ca(OH)2 + 1 H2 Just like in math, this equation may be written in a simpler way: Ca + 2 H2O Ca(OH)2 + H2 The End Let’s practice! 1. __H3PO4 + __KOH __K3PO4 + _H2O 2. __K + __B2O3 __K2O + __B 3. __Na + __NaNO3 __Na2O + __N2 4. __Na + __O2 __Na2O 5. __H3PO4 + __Mg(OH)2 __Mg3(PO4)2 + __H2O 6. __NaOH + __H2CO3 __Na2CO3 + __H2O 7. __Al(OH)3 + __H2CO3 __Al2(CO3)3 + __H2O 8. __Rb + __NO3 __Rb2O + __N2 Percent Composition • If the formula of a compound is known, it is a fairly straightforward task to determine the percent composition of each element in the compound. For example, suppose you want to calculate the percentage hydrogen and oxygen in water, H2O. First calculate the molecular mass of water: • 1 mol H2O = 2 mol H + 1 mol O • Substituting the masses involved: • 1 mol H2O = 2 (1.0079 g/mol) + 16.00 g/mol = 18.0158 g/mol • percentage hydrogen = [mass H/mass H2O] × 100 = [2(1.0079 g/mol)/18.0158 g/mol] × 100 = 11.19% H • percentage oxygen = [mass O/mass H2O] × 100= [16.00 g/mol/18.0158 g/mol] × 100 = 88.81% O • As a good check, add the percentages together. They should equal to 100% or be very close. • Determine the mass percent of each of the elements in C6H12O6 • ANSWER – Formula mass= 180.158 amu – %C =(6C atoms)(12.011 amu/atom)/(180.158 amu) x 100% = 40.002% – %H =(12H atoms)(1.008 amu/atom)/(180.158 amu) x 100% = 6.714% – %O =(6O atoms)(15.9994 amu/atom)/(180.158 amu) x 100% = 53.2846% – Total =100 001 . % Empirical Formula • The empirical formula tells us what elements are present in the compound and the simplest whole-number ratio of elements. The data may be in terms of percentage, or mass, or even moles. But the procedure is still the same: convert each to moles, divide each by the smallest number, then use an appropriate multiplier if needed. Molecular (Actual) Formula • If the actual molecular mass is known, dividing the molecular mass by the empirical formula mass gives an integer (rounded if needed) that is used to multiply each of the subscripts in the empirical formula. This gives the molecular (actual) formula, which tells which elements are in the compound and the actual number of each. Example • For example, a sample of a gas was analyzed and found to contain 2.34 g of nitrogen and 5.34 g of oxygen. The molar mass of the gas was determined to be about 90 g/mol. Answer • ( 2.34g N)x(1mol N/14.0 g N) = 0 .167 N • (1.67/1.67) = 1 N • (5.34 g O) (1 mol O/ 16.0 g O) = 0.334 mol O • (0.334 / 0.167) = 2 O • Therefore Empirical Formula = NO2 Answer • The molecular formula may be determined by dividing the actual molar mass of the compound by the empirical molar mass. In this case the empirical molar mass is 46 g/mol. • Thus (90g/mol / 46g/mol) = 1.96 which, to one significant figure, is 2. Therefore, the molecular formula is twice the empirical formula—N2O4. PRACTICE PROBLEMS 1.) What is the percent composition of N2O4? 2.) What is the empirical formula of methyl acetate 97.28 g carbon, 16.22 g hydrogen, and 86.40 g oxygen. 3.) A compound containing barium, carbon, and oxygen has the following percent composition: 69.58g Ba, 6.09g C, 24.32g O. What is the empirical formula for this compound? 4.) What is the empirical and molecular formula of Vitamin D3 if it contains 84.31% C, 11.53% H, and 4.16% O, with a molar mass of 384 g/mol? ANSWERS 1.) 30.4% N and 69.6% O 2.) C3H6O2 3.)BaCO4 4.)C27H44O (both) NOMENCLATURE • The flowchart gives explicit instructions on how to name compounds • Work backwards on the flowchart to determine the formula • Change ternarny to tertiary For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. • • • • • 1) HClO4 2) P2O5 3) NH3 4) FeSO4 5) SiO2 • • • • • 6) GaCl3 7) CoBr2 8) B2H4 9) CO 10) HClO For each of the following questions, determine whether the compound is ionic, covalent or acidic and write the appropriate formula for it. • 11) dinitrogen trioxide • 12) nitrous acid • 13) hydroiodic acid • 14) lithium acetate • 15) phosphorus trifluoride • 16) vanadium (V) oxide • 17) aluminum hydroxide • 18) Arsenous acid • 19) silicon tetrafluoride • 20) silver phosphate For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. • • • • • 1) BBr3 2) CaSO4 3) HBr 4) Cr(CO3)3 5) Ag3P • • • • • 6) H2SO3 7) VO2 8) PbS 9) CH4 10) N2O3 Write the formulas of the following chemical compounds: • 11) tetraphosphorus triselenide • 12) potassium acetate • 13) iron (II) phosphide • 14) disilicon hexabromide • 15) titanium (IV) nitrate • 16) hydrophosphoric acid • 17) copper (I) phosphate • 18) gallium sulfide • 19) tetrasulfur dinitride • 20) bromic acid