Atomic Energy
advertisement

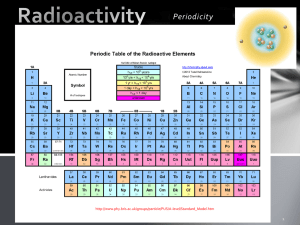
Atomic Energy Atomic Energy Agenda 1.What do you know about radiation? 2.Intro to atomic energy 3.Let’s read up on it! 4.Watch your “headsium”. 5.Alpha, Beta, Gamma 6.Project Warm-up: What do you know about radiation? Homework: Reread pages 80-87. OBJ 1: Compare alpha, beta, and gamma decay Nuclear Power http://environment.nationalgeographic.com/enviro nment/energy/great-energy-challenge/nuclearpower-quiz/ Nuclear Radiation high-energy particles and rays that are emitted by the nuclei of some atoms radioactivity: the ability to give off nuclear radiation History!! • http://www.neok12.com/Nuclear-Power.htm 3 different types of radiation. Nuclear radiation is produced through decay Radioactive decay- the process in which the nucleus of a radioactive atom releases nuclear radiation 3 types of decay: alpha beta gamma Alpha Decay • Release of an alpha particle from a nucleus • Alpha particle = 2 protons and 2 neutrons; has a mass of 4 and charge of 2+ (is identical to nucleus of a helium atom) *Large radioactive nuclei give off alpha particles to become nuclei of atoms of different elements. Reminder mass number = ___ + ___ Radium - 226 atomic number = proton number = neutron number = electron number = Conservation sum of mass number of starting material = sum of mass number of products charge is conserved . . .sum of charges of starting is always equal to sum of charges of products Radium-226 undergoes alpha decay the decay Radium 226 ----> Radon 222 Charge 88+ ----> Charge 86+ into 2 products He - 4 Charge- 2+ • • • Americium- Atomic Number : 95 Neptunium- Atomic Number: 93 Alpha Particle: 2 protons & 2 neutrons Beta Decay • Release of a beta particle from a nucleus. • Beta particle = an electron (having a charge of 1- and mass of almost 0) OR a positron (having a charge of 1+ and a mass of almost 0). *Because electrons and positrons do NOT contain protons or neutrons, mass number is 0. two types of beta decay A proton breaks down into a positron (1+) & a neutron Neutron breaks down into a proton & an electron So if there’s 1 more proton in the nucleus… • • Beta decay occurs when the neutron to proton ratio is too great in the nucleus and causes instability. In basic beta decay, a neutron is turned into a proton and an electron. The electron is then emitted. Here's a diagram of beta decay with hydrogen-3: (taken from http://library.thinkquest.org/3471/radiation_types_ body.html) • There is also positron emission when the neutron to proton ratio is too small. A proton turns into a neutron and a positron and the postiron is emitted. A positron is basically a positively charged electron. Here's a diagram of positron emission with carbon-11: Gamma Decay • Release of gamma rays from a nucleus. Occurs after alpha or beta decay as particles shift in nucleus to a more stable position. *Gamma rays alone do not cause one element to change into another. Gamma Decay • • • • • The release of gamma rays from the nucleus Energy that is released during alpha or beta decay in the form of gamma rays No mass No charge (Which is why it can penetrate through many things except… Does not cause 1 element to change into another like alpha & beta Watch Your “Headsium” page 79 in book. follow the instructions. you will need graph paper Please copy the following Trials Number of “headsium” nuclei remaining 0 100 1 2 3 4 5 6 7 8 Atomic Energy Agenda 1. Let’s watch! 2. Gamma decay 3. Watch your “headsium”. •OBJ 1: Compare alpha, beta, and gamma decay. •OBJ 2: Describe the penetrating power of the 3 types of nuclear radiation. •OBJ 3: Identify uses of radiation. •Warm-up: Take several minutes and complete the Section Review on page 84. •Homework: Read and take notes on pages 84-87. 3 different types of radiation: What does this mean? Alpha (α): could barely pass through a single sheet of paper. Deflected as a positive particle in a magnetic field. Beta (β): can pass through about 3mm of aluminum. Deflected as a negative particle in a magnetic field. Gamma (γ): can pass through several centimeters of LEAD! Not deflected in a magnetic field. • Some more history! Wilhelm Conrad Roentgen (1845-1923) Drawn to a glowing fluorescent screen on a nearby table. He determined that the fluorescence was caused by invisible rays originating from the partially evacuated glass tube he was using to study cathode rays (i.e., electrons). Surprisingly, these mysterious rays penetrated the opaque black paper wrapped around the tube. Roentgen had discovered X rays! • Antoine Henri Becquerel (1852-1908) Becquerel chose to work with was potassium uranyl sulfate,, which he exposed to sunlight and placed on photographic plates wrapped in black paper. When developed, the plates revealed an image of the uranium crystals. Becquerel initially concluded "that the phosphorescent substance in question emits radiation which penetrates paper opaque to light.“ He believed this was due to the presence of the sun's energy which was being absorbed by the uranium which then emitted X rays. Further investigation, revealed that X rays were emitted without the presence of the sun. Thus Becquerel had discovered radioactivity, the spontaneous emission of radiation by a material. • Pierre Curie (1859-1906) Marie Curie (18671934) Together, they began investigating the phenomenon of radioactivity recently discovered in uranium ore. Although the phenomenon was discovered by Henri Becquerel, the term radioactivity was coined by Marie. After chemical extraction of uranium from the ore, Marie noted the residual material to be more "active" than the pure uranium. She concluded that the ore contained, in addition to uranium, new elements that were also radioactive. This led to their discoveries of the elements of polonium and radium, but it took four more years of processing tons of ore under oppressive conditions to isolate enough of each element to determine its chemical properties. • Ernest Rutherford (1871-1937) Named and characterized the alpha particle, beta particle and proton. Even the neutron, discovered by James Chadwick, owes its name to Rutherford. The exponential equation used to calculate the decay of radioactive substances was first employed for that purpose by Rutherford and he was the first to elucidate the related concepts of the half-life and decay constant. With Frederick Soddy at McGill University, Rutherford showed that elements such as uranium and thorium became different elements (i.e., transmuted) through the process of radioactive decay. A Closer Look Atomic Energy Agenda •OBJ 4: Calculate ages of objects using halflife. 1. R. decay and Half-life •Warm-up: To determine the age of an antler 2. Watch your “headsium” which has one-fourth of its original carbon-14 unchanged, we must : multiply the number of half-lives that have passed by the length of the half life for that element (in this case 5,730 years). THUS: 2 x 5,730 yrs = 11,460 yrs. YOUR TURN: what is the age of a spear containing one-eighth its original amount of carbon-14? •Homework: Read and take notes on pages 88-94. Half-life • The amount of time it takes for one-half of the nuclei of a radioactive isotope to decay. Radiu m % Remaining 0 1620 3240 6480 12960 25920 51840 100% 50% 25% 12.5% 6.25% 3.125 1.56% % Iodine % Remaining 100% 50% 25% 12.5% 6.25% 3.125 1.56% % Uraniu m % 100% 50% 25% 12.5% 6.25% 3.125 1.56% Remaining % Radioactive Decay & the Half-life • Using the information on page 86, answer the following questions: 1. A 20 g nitrogen-13 sample is prepared for an experiment. If a scientist begins the experiment 20 minutes later, how many grams of nitrogen-13 remain? 2. At the end of the experiment, if only 2.5 g of nitrogen-13 remain, how much time has passed from the time the sample was prepared? 3. Sodium-24 has a half-life of 15 hours. There are 5 grams of Sodium24 at time 0. How long will it take to have 1.25 grams remaining? • Handout Please copy the following Trials Number of “headsium” nuclei remaining 0 100 1 2 3 4 5 6 7 8 Conclusion Take several minutes and complete the Section Review on page 87 Atomic Energy •OBJ 5: Understand the difference between nuclear fusion & fission. •OBJ 6: Identify advantages and disadvantages of energy from the nucleus. •Warm-up: Complete the Section Review on page 93. •Homework: Complete the Chapter Review on pg 98 #1-10 (this will be graded!) *Possible POP QUIZ next week! Agenda • • • Finish QuickLab questions/ graph Finish worksheet Nuclear Fusion/Fission • Nuclear Fission: when a large nucleus splits into two smaller nuclei with the release of energy • • • Some nuclei can undergo fission naturally Hit with neutrons Chain reaction • Nuclear Fusion: 2 or more nuclei with small masses join together to form a larger, more massive nucleus • The repulsion of + + has to be overcome…only way is with high temps (100,000,000 degrees) • Occurs naturally in the sun’s core Can we use nuclear fusion? • • • 1. When you wrap the Mobium in a few sheets of paper, your instruments cannot detect its radiation. What kind of radiation is Mobium probably emitting? 2. 24 hours after isolating Mobium, you determine that all but 6.25% of it has turned into iron. What is Mobium’s half-life? 3. Rounding to the nearest whole number percentage point, after how many hours will ~100% of the Mobium have turned into iron? Atomic Energy •OBJ 5: Understand the difference between nuclear fusion & fission. •OBJ 6: Identify advantages and disadvantages of energy from the nucleus. •Warm-up: Turn in Chapter Review. •Homework: Possible POP QUIZ next week! Agenda • • • Check worksheet Nuclear Fusion/Fission Domino Chain Reaction Lab The Effects of Radiation • • • • Can knock electrons out of atoms Break chemical bonds between atoms Can cause damage to living and non-living matter Radiation sickness Atomic Energy in Real Life