Virtual Model Building
advertisement

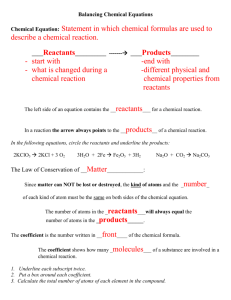
General Biology Studying Chemical Equations Name Date Period There are two stations to visit in this virtual activity. Go to my teacher page and click on the “Virtual Model Building” link. Each station is modeling a chemical reaction. If there is only one reactant or product at a station then you can leave the extra line blank in the table. REMEMBER: H = Hydrogen (white sphere) C = Carbon (black sphere) O = Oxygen (red sphere) N = Nitrogen (blue sphere) Coefficient: Used to balance an equation by telling how many molecules of a substance. Subscript: Tells how many atoms of an element in a molecule. I. Station I Reactant (include coefficient and formula) Total # of atoms of each element in the reactant (multiply coefficient x subscript for each element) Total Number of atoms in Reactants: Product (include coefficient and formula) Total # of atoms of each element in the product (multiply coefficient x subscript for each element) Total Number of atoms in Products: Write out the complete chemical equation for the reaction shown at this station. Don’t forget to use your proper coefficients and subscripts! Page 1 II. Station II Complete the table for each of the reactants at Station II. Reactant (include coefficient and formula) Total # of atoms of each element in the reactant (multiply coefficient x subscript for each element) Total Number of atoms in Reactants: Complete the table for each of the products at Station II. Product (include coefficient and formula) Total # of atoms of each element in the product (multiply coefficient x subscript for each element) Total Number of atoms in Products: Write out the complete chemical equation for the reaction shown at this station. Don’t forget to use your proper coefficients and subscripts! Page 2 The Law of Conservation of Matter states: Matter can neither be created nor destroyed. In a chemical reaction, bonds are broken, atoms are rearranged, and new bonds are formed. The same number of atoms in the reactants must be present in the products. This shows the importance of using coefficients to balance equations. Summary: a) The number of atoms of each element in the reactants [ is / is not ] the same in the as the number of atoms of each element in the products in a properly balanced equation. b) [ Coefficients / subscripts ] are used to balance an equation to tell how many molecules of a substance. c) Reactants are shown on the [ left / right ] side of a chemical equation and products are shown on the [ left / right ] side of a chemical equation. Page 3