Balancing Chemical Equations Worksheet: Steps & Rules
advertisement
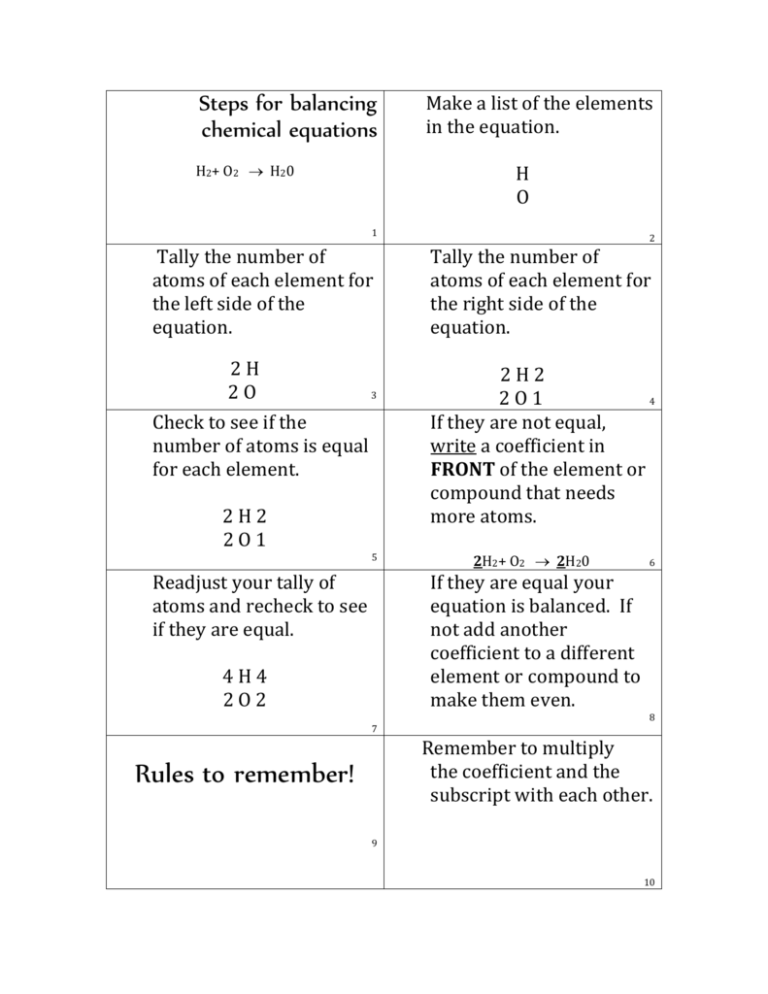
Steps for balancing chemical equations H2+ O2 H20 Make a list of the elements in the equation. H O 1 Tally the number of atoms of each element for the left side of the equation. 2H 2O 3 Check to see if the number of atoms is equal for each element. 2H2 2O1 5 Readjust your tally of atoms and recheck to see if they are equal. 2 Tally the number of atoms of each element for the right side of the equation. 2H2 2O1 4 If they are not equal, write a coefficient in FRONT of the element or compound that needs more atoms. 2H2+ O2 2H20 If they are equal your equation is balanced. If not add another coefficient to a different element or compound to make them even. 4H4 2O2 6 8 7 Remember to multiply the coefficient and the subscript with each other. Rules to remember! 9 10 Coefficients MUST be WHOLE numbers! 3CO2 NOT You can NEVER EVER add in or change a subscript. 1.5CO2 2H20 NOT H 2 O2 12 11 Coefficients are the same in math class. Distributive property! 3(x-1)= 3x-3 3H20, means that there are 6 atoms of hydrogen and 3 atoms of oxygen. Multiply the coefficient and the subscript! 13 If you see (parenthesis) Mg(OH)2 DISTRIBUTE the subscript (2) to all elements within the parenthesis. Mg 1 atom O 2 atoms H 2 atoms 14 If an element appears twice on the SAME side of an equation you will ADD them together. 3Mg(OH)2 3 Mg atoms 6 O atoms 6 H atoms CH4 + O2 CO2 + H2O So there are 1C1 4H2 2O3 15 So to balance that equation, you still place a coefficient in front of the compounds. These cards belong to: _____________________ CH4 + 2O2 CO2 + 2H2O 1C1 4 H 4 (2 +2) ( 2+2) 4 O 4 (2 +2) 16 18 17










