Dalton’s Law of Partial Pressures
advertisement
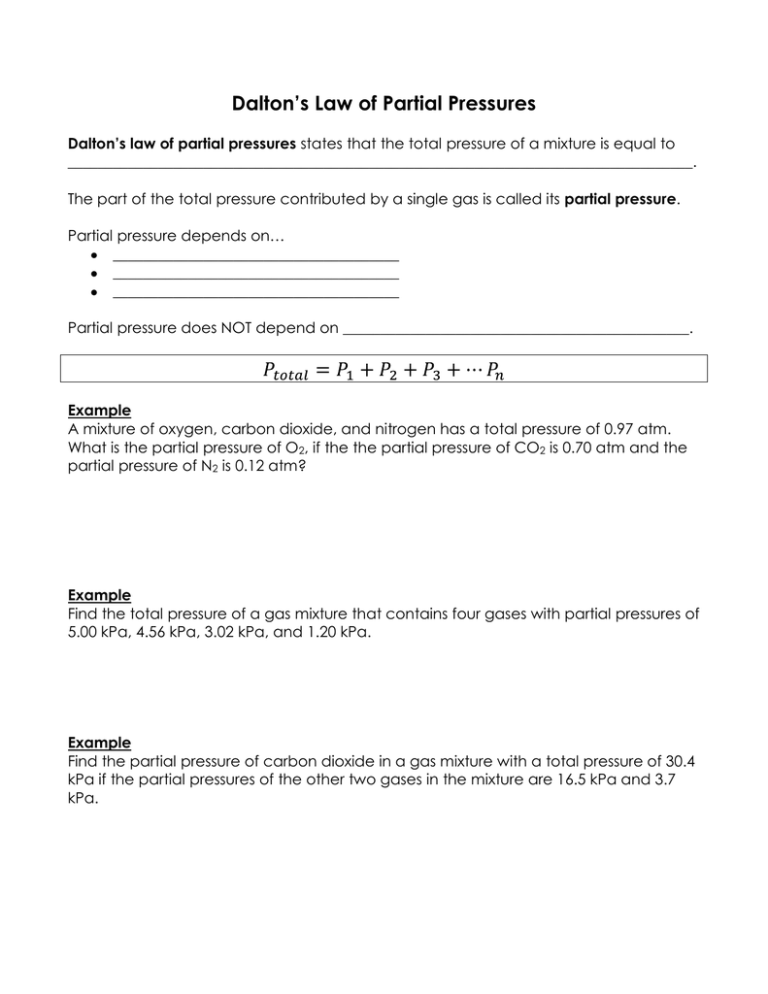
Dalton’s Law of Partial Pressures Dalton’s law of partial pressures states that the total pressure of a mixture is equal to ___________________________________________________________________________________. The part of the total pressure contributed by a single gas is called its partial pressure. Partial pressure depends on… ______________________________________ ______________________________________ ______________________________________ Partial pressure does NOT depend on ______________________________________________. Example A mixture of oxygen, carbon dioxide, and nitrogen has a total pressure of 0.97 atm. What is the partial pressure of O2, if the the partial pressure of CO2 is 0.70 atm and the partial pressure of N2 is 0.12 atm? Example Find the total pressure of a gas mixture that contains four gases with partial pressures of 5.00 kPa, 4.56 kPa, 3.02 kPa, and 1.20 kPa. Example Find the partial pressure of carbon dioxide in a gas mixture with a total pressure of 30.4 kPa if the partial pressures of the other two gases in the mixture are 16.5 kPa and 3.7 kPa. Ideal Gas Law Besides pressure, volume, and temperature, we can also use ________________________ to describe a sample of a gas. This allows for a change in the mass or amount of gas. The ideal gas law describes the physical behavior of an ideal gas in terms of ________________, ________________,________________, and _________________________. P = pressure (atm) V = volume (L) n = number of moles R= Ideal/Universal Gas Constant = 0.0821 L•atm/mol•K T = temperature (Kelvin) The value of R depends on the units being used for pressure. We will most often use 0.0821 L•atm/mol•K for our R value, but you could also use 8.314 L•kPa/mol•K when the pressure is in kPa or 62.4 L•mm Hg/mol•K when pressure is in mm Hg or torr. An ideal gas is one whose particles __________________________________________ and have no ___________________________________________. An ideal gas follows the gas laws under all condition of ____________________ and ____________________. Example Calculate the number of moles of gas contained in a 3.0 L vessel at 3.00 x 102 K with a pressure of 1.50 atm. Example What is the pressure in atmospheres of a 0.108 mol sample of helium gas at a temperature of 20.0⁰C if its volume is 0.505 L?









